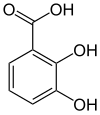
2,3-Dihydroxybenzoic acid
2,3-Dihydroxybenzoic acid is a natural phenol found in Phyllanthus acidus[2] and in the aquatic fern Salvinia molesta.
The colorless solid occurs naturally, being formed via the shikimate pathway.
It is incorporated into various siderophores, which are molecules that strongly complex iron ions for absorption into bacteria.
2,3-DHB consists of a catechol group, which upon deprotonation binds iron centers very strongly, and the carboxylic acid group by which the ring attaches to various scaffolds through amide bonds.
A famous high affinity siderophore is enterochelin, which contains three dihydroxybenzoyl substituents linked to the depsipeptide of serine.