MCPA
[2][3] In 1936 investigations began at ICIs Jealott's Hill research center into the effects of auxins on plant growth looking specifically for a way to kill weeds without harming crops such as wheat and oats.
William Templeman found that when indole-3-acetic acid (IAA), the naturally occurring auxin, was used at high concentrations, it could stop plant growth.
All four groups were subject to wartime secrecy laws and did not follow the usual procedures of publication and patent disclosure, although ICI did file an application relating to both MCPA and 2,4-D on 7 April 1941 in the UK.
[5] The first publications about this group of herbicides were by other workers who were not the original inventors: the precise sequence of discovery events has been discussed.
Uncontrolled, unsustainable growth ensues, causing stem curl-over, leaf withering, and eventual plant death.
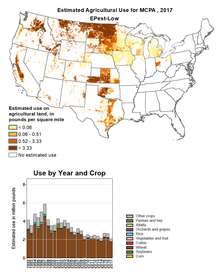
It is currently classified as a restricted use pesticide in the United States: its use is mapped by the US Geological Survey, whose data show consistent use from 1992, with a small recent decline in the ten years to 2017, the latest date for which figures are available.
One formulation is described by its manufacturer as "designed for specific markets that require the safest possible phenoxy product, primarily for use in the Pacific Northwest".
[10] Though not extremely toxic,[11] it has been determined that MCPA can form complexes with metal ions and thereby increase their bioavailability,[12] and there is also work being done to utilize this ability.
MCPA herbicide is usually sprayed to the soil surface and plant leaves in its water solution, sometimes with additional surfactant.
However, the concentration of MCPA and MCP detected in water and soil are lower than the predicted no-effect levels of all environmental compartments, and considered to present low potential risk.
[20] In the general pH range of aqueous environments, the MCPA-metal complex has higher solubility than metal ions.
Recent studies have demonstrated that biological degradation of MCPA is enzymatically catalyzed by an α-ketoglutarate-dependent dioxygenase encoded by the tfdA gene of soil microorganisms.