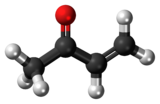
Methyl vinyl ketone
Methyl vinyl ketone (MVK, IUPAC name: butenone) is the organic compound with the formula CH3C(O)CH=CH2.
Similarly it is prepared by the Mannich reaction involving diethylammonium chloride and acetone, which produces the Mannich adduct:[2][3] Heating this ammonium salt releases the ammonium chloride and the MVK:[3] MVK can act as an alkylating agent because it is an effective Michael acceptor.
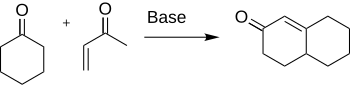
It gained early attention for its use in the Robinson annulation, a method useful in the preparation of steroids: Its alkylating ability is both the source of its high toxicity and the feature that makes it a useful intermediate in organic synthesis.
[2] MVK is an intermediate in the synthesis of some pharmaceutical drugs including etorphine, buprenorphine, tolquinzole, butaclamol, and etretinate.
MVK is extremely hazardous upon inhalation causing coughing, wheezing and shortness of breath even at low concentrations.