Abacavir
[8] Common side effects include vomiting, insomnia (trouble sleeping), fever, and feeling tired.
[5] Other common side effects include loss of appetite, headache, nausea (feeling sick), diarrhea, rash, and lethargy (lack of energy).
[8] Abacavir is in the NRTI class of medications, which work by blocking reverse transcriptase, an enzyme needed for HIV virus replication.
[3][4] Common adverse reactions include nausea, headache, fatigue, vomiting, diarrhea, Anorexia (symptom) (loss of appetite), and insomnia (trouble sleeping).
Rare but serious side effects include hypersensitivity reaction such as rash, elevated AST and ALT, depression, anxiety, fever/chills, URI, lactic acidosis, hypertriglyceridemia, and lipodystrophy.
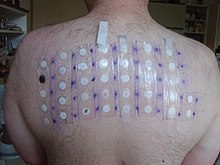
[13] Hypersensitivity to abacavir is strongly associated with a specific allele at the human leukocyte antigen B locus namely HLA-B*5701.
On 1 March 2011, the FDA informed the public about an ongoing safety review of abacavir and a possible increased risk of heart attack associated with the drug.
Abacavir binds with high specificity to the HLA-B*5701 protein, changing the shape and chemistry of the antigen-binding cleft.
This results in a change in immunological tolerance and the subsequent activation of abacavir-specific cytotoxic T cells, which produce a systemic reaction known as abacavir hypersensitivity syndrome.
Abacavir is metabolized primarily through the enzymes alcohol dehydrogenase and glucuronyl transferase to an inactive carboxylate and glucuronide metabolites.
[37] Robert Vince and Susan Daluge along with Mei Hua, a visiting scientist from China, developed the medication in the '80s.