Antifreeze
Common antifreezes also increase the boiling point of the liquid, allowing higher coolant temperature.
The purpose of antifreeze is to prevent a rigid enclosure from bursting due to expansion when water freezes.
Commercially, both the additive (pure concentrate) and the mixture (diluted solution) are called antifreeze, depending on the context.
Most if not all commercial antifreeze formulations intended for use in heat transfer applications include anti-corrosion and anti-cavitation agents (that protect the hydraulic circuit from progressive wear).
Freezing and boiling points are colligative properties of a solution, which depend on the concentration of dissolved substances.
Low molecular weight organic compounds tend to have melting points lower than water, which makes them suitable for use as antifreeze agents.
When used in an automotive context, corrosion inhibitors are added to help protect vehicles' radiators, which often contain a range of electrochemically incompatible metals (aluminum, cast iron, copper, brass, solder, etc.).
The Volkswagen Group has been particularly committed to the development of coolants and their standards (VW TL 774 ) in collaboration with Haertol Chemie from Magdeburg.
However, EGW solutions formulated for the automotive industry often have silicate based rust inhibitors that can coat and/or clog heat exchanger surfaces.
Many formulations have corrosion inhibitors, and it is expected that these chemicals will be replenished (manually or under automatic control) to keep expensive piping and equipment from corroding.
Antifreeze proteins refer to chemical compounds produced by certain animals, plants, and other organisms that prevent the formation of ice.
[5][6] Cryoprotectants are commonly used in cryobiology to prevent or inhibit freezing in sperm, blood, stem cells, plant seeds, etc.
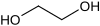
Ethylene glycol solutions first became available in 1926 and were marketed as "permanent antifreeze" since the higher boiling points provided advantages for summertime use as well as during cold weather.
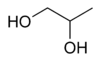
It is used as antifreeze where ethylene glycol would be inappropriate, such as in food-processing systems or in water pipes in homes where incidental ingestion may be possible.
For example, the U.S. FDA allows propylene glycol to be added to a large number of ultra-processed foods, including ice cream, frozen custard, salad dressings, and baked goods, and it is commonly used as the main ingredient in the "e-liquid" used in electronic cigarettes.
Maintenance of systems using glycol solution includes regular monitoring of freeze protection, pH, specific gravity, inhibitor level, color, and biological contamination.
In the absence of inhibitors, propylene glycol can react with oxygen and metal ions, generating various compounds including organic acids (e.g., formic, oxalic, acetic).
[15][16] Volkswagen introduced G13 (TL 774-G) antifreezes containing glycerol in 2008, marketed as better for the environment due to its low toxicity and reduced CO2 emissions.
[20] Most commercial antifreeze formulations include corrosion inhibiting compounds, and a colored dye (commonly a fluorescent green, red, orange, yellow, or blue) to aid in identification.
In warmer or colder areas, weaker or stronger dilutions are used, respectively, but a range of 40%/60% to 60%/40% is frequently specified to ensure corrosion protection, and 70%/30% for maximum freeze prevention down to −84 °F (−64 °C).
For simplicity, most automotive manufacturers recommend periodic complete replacement of engine coolant, to simultaneously renew corrosion inhibitors and remove accumulated contaminants.
One of the anti-corrosion components presented as sodium or potassium 2-ethylhexanoate and ethylhexanoic acid is incompatible with nylon 6,6 and silicone rubber, and is a known plasticizer.
[29] GM (Motors Liquidation Company) filed for bankruptcy in 2009, which tied up the outstanding claims until a court determines who gets paid.
[31] DEX-COOL antifreeze uses two inhibitors: sebacate and 2-EHA (2-ethylhexanoic acid), the latter which works well with the hard water found in the United States, but is a plasticizer that can cause gaskets to leak.
[23] According to internal GM documents,[31] the ultimate culprit appears to be operating vehicles for long periods of time with low coolant levels.
[21] This dye fluoresces bright green when illuminated by blue or UV light from daylight or testing lamps.
The main ingredient which makes it dangerous is ethylene glycol, which, when ingested, is metabolized in the liver into various intermediate substances, which then get turned into oxalic acid.
Common symptoms of poisoning are vomiting, confusion, Abdominal pain, Agitation, ataxia and hematuria.