Bacteriochlorophyll
Organisms that contain bacteriochlorophyll conduct photosynthesis to sustain their energy requirements, but the process is anoxygenic and does not produce oxygen as a byproduct.
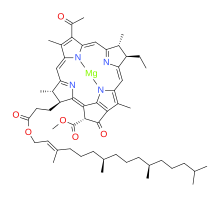
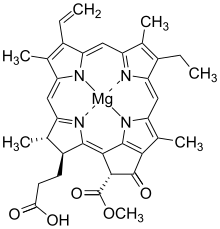
[4] Bacteriochlorophylls c to f occur in the form of closely related homologs with different alkyl groups attached to pyrrole rings B and C and are illustrated above in their simplest versions, esterified with the sesquiterpene alcohol farnesol.
[8] There are a large number of known bacteriochlorophylls[4][9] but all have features in common since the biosynthetic pathway involves chlorophyllide a (Chlide a) as an intermediate.
The bacteriochlorin-cored BChls a, b, g require a unique step to reduce the double bound between C7 and C8, which is performed by Chlorophyllide a reductase (COR).
[9] Isobacteriochlorins, in contrast, are biosynthesised from uroporphyrinogen III in a separate pathway that leads, for example, to siroheme, cofactor F430 and cobalamin.