Benzoic acid
Benzoic acid (/bɛnˈzoʊ.ɪk/) is a white (or colorless) solid organic compound with the formula C6H5COOH, whose structure consists of a benzene ring (C6H6) with a carboxyl (−C(=O)OH) substituent.
Benzoic acid occurs naturally in many plants[9] and serves as an intermediate in the biosynthesis of many secondary metabolites.
The dry distillation of gum benzoin was first described by Nostradamus (1556), and then by Alexius Pedemontanus (1560) and Blaise de Vigenère (1596).
[10] Justus von Liebig and Friedrich Wöhler determined the composition of benzoic acid.
In 1875 Salkowski discovered the antifungal properties of benzoic acid, which explains the preservation of benzoate-containing cloudberry fruits.
[12][disputed – discuss] Benzoic acid is produced commercially by partial oxidation of toluene with oxygen.
For this reason, benzoic acid for human consumption was obtained by dry distillation of gum benzoin.
Benzoic acid is mainly consumed in the production of phenol by oxidative decarboxylation at 300−400 °C:[22] The temperature required can be lowered to 200 °C by the addition of catalytic amounts of copper(II) salts.
[27] Benzoic acid is a constituent of Whitfield's ointment which is used for the treatment of fungal skin diseases such as ringworm and athlete's foot.
Ripe fruits of several Vaccinium species (e.g., cranberry, V. vitis macrocarpon; bilberry, V. myrtillus) contain as much as 0.03–0.13% free benzoic acid.
Among animals, benzoic acid has been identified primarily in omnivorous or phytophageous species, e.g., in viscera and muscles of the rock ptarmigan (Lagopus muta) as well as in gland secretions of male muskoxen (Ovibos moschatus) or Asian bull elephants (Elephas maximus).
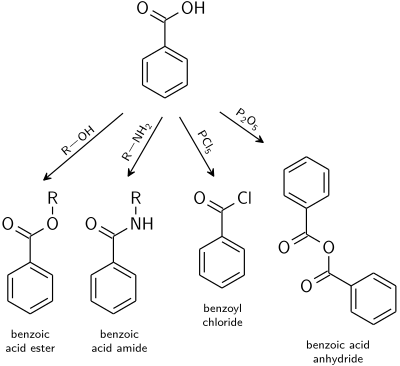
Electrophilic aromatic substitution reaction will take place mainly in 3-position due to the electron-withdrawing carboxylic group; i.e. benzoic acid is meta directing.
[40] For humans, the World Health Organization's International Programme on Chemical Safety (IPCS) suggests a provisional tolerable intake would be 5 mg/kg body weight per day.
[32] In Taipei, Taiwan, a city health survey in 2010 found that 30% of dried and pickled food products had benzoic acid.