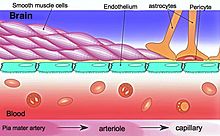
Blood–brain barrier
[2] This system allows the passage of some small molecules by passive diffusion, as well as the selective and active transport of various nutrients, ions, organic anions, and macromolecules such as glucose and amino acids that are crucial to neural function.
[8] The BBB results from the selectivity of the tight junctions between the endothelial cells of brain capillaries, restricting the passage of solutes.
[14] Measurement of brain uptake of various blood-borne solutes showed that newborn endothelial cells were functionally similar to those in adults,[15] indicating that a selective BBB is operative at birth.
[17] The blood–brain barrier acts effectively to protect brain tissue from circulating pathogens and other potentially toxic substances.
[22][24] Permeable capillaries of the sensory CVOs (area postrema, subfornical organ, vascular organ of the lamina terminalis) enable rapid detection of circulating signals in systemic blood, while those of the secretory CVOs (median eminence, pineal gland, pituitary lobes) facilitate transport of brain-derived signals into the circulating blood.
[25][26] The permeable capillary zone shared between the median eminence and hypothalamic arcuate nucleus is augmented by wide pericapillary spaces, facilitating bidirectional flow of solutes between the two structures, and indicating that the median eminence is not only a secretory organ, but may also be a sensory organ.
[29][30] In its neuroprotective role, the blood–brain barrier functions to hinder the delivery of many potentially important diagnostic and therapeutic agents to the brain.
[29] To overcome this problem some peptides able to naturally cross the BBB have been widely investigated as a drug delivery system.
Modalities for drug delivery to the brain in unit doses through the BBB entail its disruption by osmotic means, or biochemically by the use of vasoactive substances, such as bradykinin,[32] or even by localized exposure to high-intensity focused ultrasound (HIFU).
A 1898 study observed that low-concentration "bile salts" failed to affect behavior when injected into the blood of animals.
[43] Two years later, Max Lewandowsky may have been the first to coin the term "blood–brain barrier" in 1900, referring to the hypothesized semipermeable membrane.
Due to the language barrier between her publications and English-speaking scientists, this could have made her work a lesser-known origin of the term.
[44] However, in a later experiment in 1913, Edwin Goldmann (one of Ehrlich's students) injected the dye directly into the cerebrospinal fluid of animal brains.