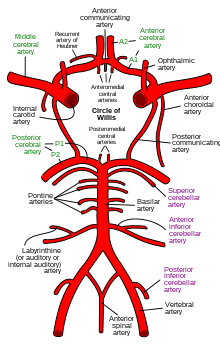
Cerebral circulation
The rate of cerebral blood flow in an adult human is typically 750 milliliters per minute, or about 15% of cardiac output.
Veins carry "used or spent" blood back to the heart, to remove carbon dioxide, lactic acid, and other metabolic products.
The neurovascular unit regulates cerebral blood flow so that activated neurons can be supplied with energy in the right amount and at the right time.
Sudden intense accelerations change the gravitational forces perceived by bodies and can severely impair cerebral circulation and normal functions to the point of becoming serious life-threatening conditions.
In the neck, the jugular veins parallel the upward course of the carotid arteries and drain blood into the superior vena cava.
Endothelial cells begin to express P-glycoprotein, a crucial efflux transporter that helps protect the brain by expelling harmful substances.
Additionally, VSMCs, which initially populate the arterial network, start to express contractile proteins such as smooth muscle actin (SMA) and myosin-11, transforming VSMCs into contractile cells capable of regulating blood vessel tone and cerebral blood flow.
The expression of Myh11 in VSMCs acts as a developmental switch, with significant upregulation occurring from birth to the age of 2 to 5 years.
[10][13] Too much blood (a clinical condition of a normal homeostatic response of hyperemia)[1] can raise intracranial pressure (ICP), which can compress and damage delicate brain tissue.
Medical professionals must take steps to maintain proper CBF in patients who have conditions like shock, stroke, cerebral edema, and traumatic brain injury.
[17] This is why small alterations in respiration pattern can cause significant changes in global CBF, specially through PaCO2 variations.
Arterial spin labeling (ASL), phase contrast magnetic resonance imaging (PC-MRI), and positron emission tomography (PET) are neuroimaging techniques that can be used to measure CBF.