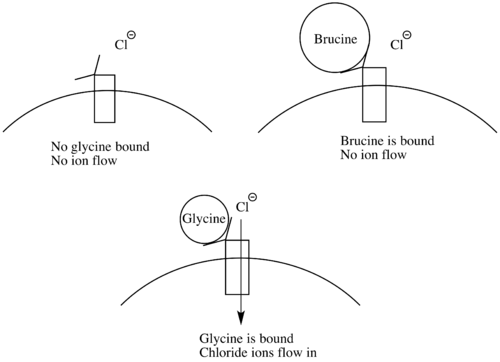
Brucine
Fisher first reported its use as a resolving agent in 1899, and it was the first natural product used as an organocatalyst in a reaction resulting in an enantiomeric enrichment by Marckwald, in 1904.
[8] If formic acid is added to a mixture of brucine and potassium nitrate, its color instantly turns red.
[14] One of the most famous cultural references to brucine occurs in The Count of Monte Cristo, the novel by French author Alexandre Dumas.
In a discussion of mithridatism, Monte Cristo states: “Well, suppose, then, that this poison was brucine, and you were to take a milligramme the first day, two milligrams the second day, and so on…at the end of a month, when drinking water from the same carafe, you would kill the person who drank with you, without your perceiving…that there was any poisonous substance mingled with this water.”[15] Brucine is also mentioned in the 1972 version of The Mechanic, in which the hitman Steve McKenna betrays his mentor, ageing hitman Arthur Bishop, using a celebratory glass of wine spiked with brucine, leaving Bishop to die of an apparent heart attack.
[16] Such fictions run contrary to reality in the very properties which make brucine useful as a denaturant, and useless as a covert poison.