COVID-19 rapid antigen test
They are quick to implement with minimal training, cost a fraction of other forms of COVID-19 testing, and give users a result within 5–30 minutes.
[22] However, many experts were unsure of this approach believing that "rapid tests are not the solution to restart normal life"[23] but might be used in conjunction with other infection control techniques.
As part of the UK's accelerated technology evaluation of lateral flow, within 24 hours Public Health England laboratories were able to confirm that RATs in global development were not affected (i.e., that they could identify the new variant).
[33] Later, researchers found that introducing fruit juices, alcoholic beverages, bottled water, and other products directly into an Abbott Panbio COVID-19 RAT without the manufacturer's recommended buffer solution produced false positives.
A modelling study in Canada estimated that half the deaths in care homes in British Columbia in 2020 could have been prevented if rapid testing had been available.
[36] On 24 February 2022, Health Canada issued a public advisory warning that there had been a recent increase in calls to poison control centers associated with RATs, which contained the preservatives[37] sodium azide and ProClin.
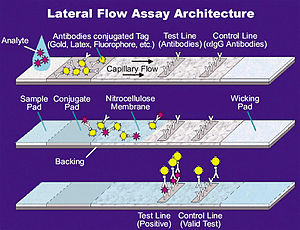
[39]: Table S1 [41] For an LFT, a liquid sample (such as from a nasal swab) is placed on a pad at one end of a porous paper-like strip.
First, at a "conjugate pad," soluble antibodies with gold nanoparticles are picked up by the liquid, attaching to any SARS-CoV-2 antigens present in the sample.
[41][42][43] In May 2020, the US Food and Drug Administration (FDA) issued the first emergency use authorization (EUA) for a COVID-19 RAT, the Sofia 2 test manufactured by Quidel.
[44] In August 2020, the United States Department of Health and Human Services announced that the US government would be purchasing 150 million BinaxNOW RATs produced by Abbott Laboratories "to expand strategic, evidence-based testing.
[46] By September 2020, it was reported that the United Kingdom's Moonshot program would be investing £100 billion to systematically assess, develop and implement new technologies for COVID-19 testing, including RATs for COVID-19.
[47] In that month, the World Health Organization (WHO) released interim guidance on COVID-19 "antigen-detecting rapid diagnostic tests" (Ag-RDTs) including recommendations for their use, selection, and implementation.
[48] The interim guidance noted that Ag-RDTs were easier to implement and less expensive than nucleic acid amplification tests (NAATs).
[48] The World Health Organization (WHO) recommended the use of Ag-RDTs in outbreaks, in monitoring disease trends, and in early identification of cases.
[52] One of the early large studies of COVID-19 lateral flow devices (LFDs) was completed by Public Health England and University of Oxford, with a preliminary report released in November 2020.
On 6 November 2020, the Prime Minister, Boris Johnson, started city-wide screening of Liverpool as part of the accelerated technology evaluation.
[66][67] Global efforts to step up evaluations of rapid tests were initiated by the World Health Organization (WHO) Emergencies Department who launched a major rapid diagnostic test implementation project on 10 November 2020, aided by agreement from the Bill and Melinda Gates Foundation that limited costs for low- and middle-income countries.
Global piloting of rapid tests was now common place in schools in Canada,[75] travel hubs in Indonesia,[76] and across India.
[77] Professor William A. Haseltine of Harvard said that "rapid, self-administered testing could stem the ever-surging tide of disease and death.
[82] In the UK in December 2020, the Medicines and Healthcare products Regulatory Agency approved the Innova rapid test for self-testing of asymptomatic people.
[84] Noting the ability to identify cases more rapidly, and considering the ensuing escalation in cases in Europe, the European Commission (EC) met in December 2020 and developed a common European framework for "use, validation and mutual recognition of rapid tests", committing 100 million euros for the purchase of tests from Roche and Abbott.
[85] Stella Kyriakides, commissioner for Health and Food Safety, said "Rapid antigen tests offer us speed, reliability and quick responses to isolate COVID cases.
"[89] A week after the FDA's warning about the Innova test, UK's Medicines and Healthcare products Regulatory Agency (MHRA) cleared the rapid diagnostic's use and extended its authorization.
[94] In October 2024, the US Food and Drug Administration granted marketing authorization for the Healgen Rapid Check COVID-19/Flu A&B Antigen Test.
[95] The test, authorized for use without a prescription, is for use by individuals experiencing respiratory symptoms and uses a nasal swab sample to deliver at-home results in approximately 15 minutes for COVID-19 and influenza (flu).
[96] A 2021 study from Germany found that monitoring health care workers exposed to COVID-19 with RATs saves money compared with sending them into quarantine.
[97] A 2021 study concluded that if the US is willing to pay $100,000 per year of life lost averted, then weekly or monthly testing of the population using RATs is likely to be cost-effective.