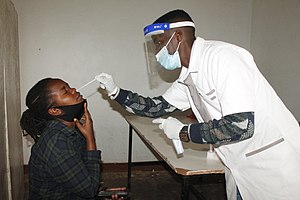
COVID-19 testing
[21] Other methods used in molecular tests include CRISPR, isothermal nucleic acid amplification, digital polymerase chain reaction, microarray analysis, and next-generation sequencing.
[21] Polymerase chain reaction (PCR) is a process that amplifies (replicates) a small, well-defined segment of DNA many hundreds of thousands of times, creating enough of it for analysis.
The Minimum Information for Publication of Quantitative Real-Time PCR Experiments (MIQE) guidelines propose the term RT-qPCR,[25] but not all authors adhere to this.
[44][45][46] On 15 August 2020, the US FDA granted an emergency use authorization for a saliva test developed at Yale University that gives results in hours.
[5] They can also be used to determine how much antibody is contained in a unit of convalescent plasma, for COVID-19 treatment, or to verify if a given vaccine generates an adequate immune response.
[91] Typical visible features on CT initially include bilateral multilobar ground-glass opacities with a peripheral or posterior distribution.
Patients who had low-grade symptoms and high body temperatures revealed significant lung indications on their chest computed tomography scans.
The equipment needed for computed tomography scans is not available in most hospitals, making it not as effective as some other tools used for detection of the coronavirus disease.
[citation needed] In May 2021, Reuters reported that Dutch researchers at Wageningen University had shown that trained bees could detect the virus in infected samples in seconds and this could benefit countries where test facilities are in short supply.
[102] A two-month study by the Necker-Cochin hospital Paris in conjunction with the French national veterinary school reported in May 2021 that dogs were more reliable than current lateral flow tests.
[103] Researchers in Paris in March 2022 reported in a preprint not yet peer-reviewed that trained dogs were very effective for rapidly detecting the presence of SARS-Cov2 in people, whether displaying symptoms or not.
[106] In January 2020, scientists from China published the first genetic sequences of SARS-CoV-2 via virological.org,[108] a "hub for prepublication data designed to assist with public health activities and research".
[124][125][126] Pool testing was then adopted in Israel, Germany, Ghana[127][128][129] South Korea,[130] Nebraska,[131] China[132] and the Indian states of Uttar Pradesh,[133] West Bengal,[134] Punjab,[135] Chhattisgarh[136] and Maharashtra.
[150] As of August 2020, the WHO recognizes wastewater surveillance of SARS-CoV-2 as a potentially useful source of information on the prevalence and temporal trends of COVID-19 in communities, while highlighting that gaps in research such as viral shedding characteristics should be addressed.
[153][154][155][156][157] This may prove particularly useful once large shares of regional populations are vaccinated or recovered and do not need to conduct rapid tests while in some cases being infectious nevertheless.
Tests are available that look for viral RNA using either polymerase chain reaction (PCR) or loop-mediated isothermal amplification (LAMP) technology.
Tests developed in China, France, Germany, Hong Kong, Japan, the United Kingdom, and the US targeted different parts of the viral genome.
[160] The assay amplifies a unique region of the virus's RdRp gene; the resulting copies are then detected with "fluorescently-labeled molecular beacons".
Quidel's "Sofia 2 SARS Antigen FIA"[66][163] is a lateral flow test that uses monoclonal antibodies to detect the virus's nucleocapsid (N) protein.
The FDA inspected Innova facilities in California in March and April 2021, and found inadequate quality assurance of tests manufactured in China.
The review called for higher quality studies assessing accuracy with reference to a standard of "RT-PCR performed on at least two consecutive specimens, and, when feasible, includ[ing] viral cultures.
"[174][175] CEBM researchers have called for in-hospital 'case definition' to record "CT lung findings and associated blood tests"[176] and for the WHO to produce a "protocol to standardise the use and interpretation of PCR" with continuous re-calibration.
[188] In July 2020, Dr. Anthony Fauci of the US NIH indicated that positive results obtained from RT-PCR tests run at more than 35 cycles were almost always "just dead nucleotides".
[192] The University of Oxford's Centre for Evidence-Based Medicine (CEBM) has pointed to mounting evidence[194][195] that "a good proportion of 'new' mild cases and people re-testing positives via RT-PCR after quarantine or discharge from hospital are not infectious, but are simply clearing harmless virus particles which their immune system has efficiently dealt with", and have called for "an international effort to standardize and periodically calibrate testing".
[204][207] On 3 April, those with positive tests were legally permitted to recuperate at home or in a hotel if they had asymptomatic or mild illness, ending the hospital bed shortage.
The government considered the mass test would significantly assist in controlling the virus and avoid a lockdown and may repeat the exercise at a later date.
Testing capacity, largely in private sector labs, was built up over several years by the South Korean government in the early 2000s.
Denmark's Minister of Transport, Benny Engelbrecht said that they were taking time to ensure that the negative tests of travelers from the Emirates were a real screening carried out appropriately.
On 21 March New York City health officials directed medical providers to test only those entering the hospital, for lack of PPE.
[253] In 2020, Nevada received a donation of 250,000 Covid testing kits, which were a product of China's leading genetics company, BGI Group.






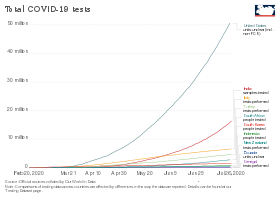

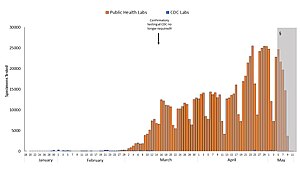
Blue: CDC lab
Orange: Public health lab
Gray: Data incomplete due to reporting lag
Not shown: Testing at private labs; total exceeded 100,000 per day by 27 March. [ 141 ]





