Calcium citrate
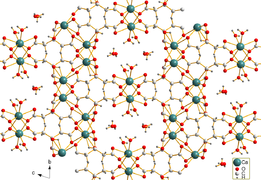
Needle-shaped crystals of tricalcium dicitrate tetrahydrate [Ca3(C6H5O7)2(H2O)2]·2H2O were obtained by hydrothermal synthesis.
The crystal structure comprises a three-dimensional network in which eightfold coordinated Ca2+ cations are linked by citrate anions and hydrogen bonds between two non-coordinating crystal water molecules and two coordinating water molecules.
[2] The citric acid in the broth solution is neutralized by limewater, precipitating insoluble calcium citrate.
This is then filtered off from the rest of the broth and washed to give clean calcium citrate.
This is mainly due to the changes related to where calcium absorption occurs in the digestive tract of these individuals.