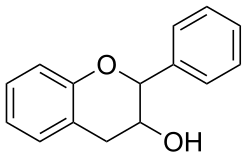
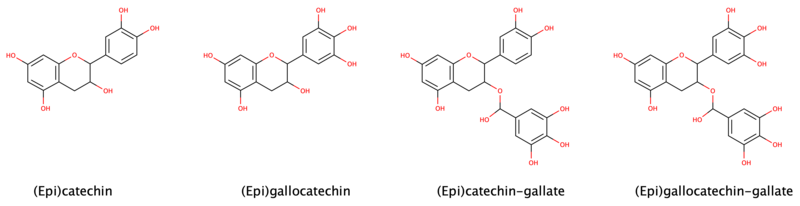
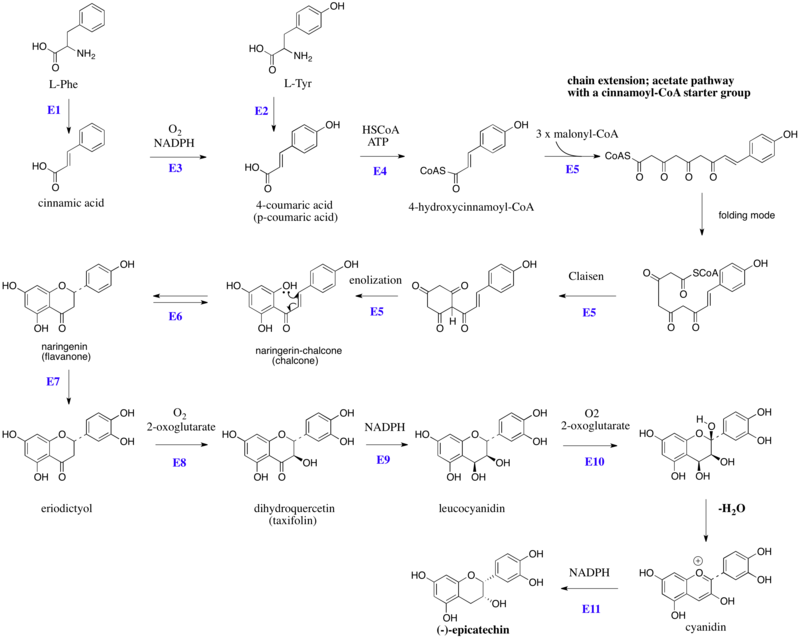
Flavan-3-ol
Early use of the term bioflavonoid was imprecisely applied to include the flavanols, which are distinguished by absence of ketone(s).
Heating catechin past its point of decomposition releases pyrocatechol (also called catechol), which explains the common origin of the names of these compounds.
These enzyme do not use ACPSs, but instead employ coenzyme A esters and have a single active site to perform the necessary series of reactions, e.g. chain extension, condensation, and cyclization.
The colonic microbiome has also an important role in the metabolism of flavan-3-ols and they are catabolized to smaller compounds such as 5-(3′/4′-dihydroxyphenyl)-γ-valerolactones and hippuric acid.
[18] As catechins in green tea extract can be hepatotoxic, Health Canada and EFSA have advised for caution,[19] recommending intake should not exceed 800 mg per day.
[21][22] In 2015, the European Commission approved a health claim for cocoa solids containing 200 mg of flavanols, stating that such intake "may contribute to maintenance of vascular elasticity and normal blood flow".
[23][24] As of 2022, food-based evidence indicates that intake of 400–600 mg per day of flavan-3-ols could have a small positive effect on cardiovascular biomarkers.