Cathodic protection
For structures such as long pipelines, where passive galvanic cathodic protection is not adequate, an external DC electrical power source is used to provide sufficient current.
Cathodic protection was first described by Sir Humphry Davy in a series of papers presented to the Royal Society[2] in London in 1824.
In 1834, Faraday discovered the quantitative connection between corrosion weight loss and electric current and thus laid the foundation for the future application of cathodic protection.
It then reverts to a lower sacrificial current, while harmful negative chloride ions migrate away from the steel and towards the positive anode.
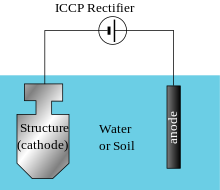
Unlike impressed current cathodic protection (ICCP) systems, steel constant polarization is not the goal, rather the restoration of the environment.
Polarization of the target structure is caused by the electron flow from the anode to the cathode, so the two metals must have a good electrically conductive contact.
These include high silicon, cast iron, graphite, mixed metal oxide (MMO), platinum and niobium coated wire and other materials.
Cathodic protection transformer-rectifier units are often custom manufactured and equipped with a variety of features, including remote monitoring and control, integral current interrupters and various type of electrical enclosures.
Some cathodic protection transformer-rectifier units are designed with taps on the transformer windings and jumper terminals to select the voltage output of the ICCP system.
Cathodic protection transformer-rectifier units for water tanks and used in other applications are made with solid state circuits to automatically adjust the operating voltage to maintain the optimum current output or structure-to-electrolyte potential.
[17] Analog or digital meters are often installed to show the operating voltages (DC and sometimes AC) and current output.
Indeed, the electrons sent by the imposed current anode (composed of titanium and covered with MMO) prevents the inside of the tank from rusting.
Cathodic protection on ships is often implemented by galvanic anodes attached to the hull and ICCP for larger vessels.
Since ships are regularly removed from the water for inspections and maintenance, it is a simple task to replace the galvanic anodes.
[25] Smaller vessels, with non-metallic hulls, such as yachts, are equipped with galvanic anodes to protect areas such as outboard motors.
The anode cables are introduced into the ship via a compression seal fitting and routed to the DC power source.
Because of the wide variety of structure geometry, composition, and architecture, specialized firms are often required to engineer structure-specific cathodic protection systems.
This makes for a more complicated system and usually an automatically controlled DC power source is used, possibly with an option for remote monitoring and operation.
[41] A common application of internal cathodic protection is water storage tanks and power plant shell and tube heat exchangers.
"[49] Under section 74.01(1) (b) of the Competition Act Canada, no performance claims about a product or its effectiveness can be done unless it can be proven that they are based on adequate and proper tests.
[53] The test results, as reported to and validated by the Competition Bureau,[54] demonstrated that the Auto Saver module being tested was able to cause a shift, in the negative direction, in the electrochemical corrosion potential of the iron in the steel panels, proving the attainment of cathodic protection and the resulting slowdown of the oxidation process of the iron (rust formation).
[56] A second company, Canadian Auto Preservation Inc., was also able to satisfy the Competition Bureau proving that the testing of its Electromagnetically Induced Corrosion Control Technology (EICCT) was adequate and proper.
[57] The testing of that module, which relied on a methodology very similar to that used by Auto Saver, also produced a shift, in the negative direction, in the electrochemical corrosion potential of the iron galvanized automotive steel panels, consistent with the attainment of cathodic protection.
[58][59] A peer review article alluding to the efficacy of the Final Coat technology in inhibiting corrosion on automobiles was published in 2017.
[60] The results achieved by both these electronic corrosion inhibitor devices point to the need for further research and testing in order to better understand how these devices are able to generate a shift in the potential of the metal panels, i.e., a cathodic effect, in the absence of a continuous electrolytic path required to close the electrical circuit between the positive and the negative poles, in accordance with accepted principles of cathodic protection.
Cathodic disbonding occurs rapidly in pipelines that contain hot fluids because the process is accelerated by heat flow.
[citation needed] Effectiveness of cathodic protection (CP) systems on steel pipelines can be impaired by the use of solid film backed dielectric coatings such as polyethylene tapes, shrinkable pipeline sleeves, and factory applied single or multiple solid film coatings.
Newly issued USDOT regulation Title 49 CFR 192.112, in the section for Additional design requirements for steel pipe using alternative maximum allowable operating pressure requires that "The pipe must be protected against external corrosion by a non-shielding coating" (see coatings section on standard).
[71] The main center for cathodic protection certification in France and some French language countries is CEFRACOR.
[74][75] Three different bodies will provide cathodic protection certificates based on ISO 15257: APCERT, CICPND, and RINA.