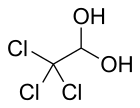
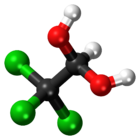
Chloral hydrate
Manufacturers contend that such "legacy drugs", by virtue of the fact that they have been prescribed for decades, have gained a history of safety and efficacy.
It was also formerly used in veterinary medicine as a general anesthetic but is not considered acceptable for anesthesia or euthanasia of small animals due to adverse effects.
In this synthesis, chloral hydrate reacts with aniline and hydroxylamine to give a condensation product which cyclicizes in sulfuric acid to give the target compound:[12] Moreover, chloral hydrate is used as a reagent for the deprotection of acetals, dithioacetals and tetrahydropyranyl ethers in organic solvents.
[14] Chloral hydrate is also an ingredient used for Hoyer's solution, a mounting medium for microscopic observation of diverse plant types such as bryophytes, ferns, seeds, and small arthropods (especially mites).
Chloral hydrate is an ingredient used to make Melzer's reagent, an aqueous solution that is used to identify certain species of fungi.
[17] Acute overdosage is often characterized by nausea, vomiting, confusion, convulsions, slow and irregular breathing, cardiac arrhythmia, and coma.
The plasma, serum or blood concentrations of chloral hydrate and/or trichloroethanol, its major active metabolite, may be measured to confirm a diagnosis of poisoning in hospitalized patients or to aid in the forensic investigation of fatalities.
Accidental overdosage of young children undergoing simple dental or surgical procedures has occurred.
In basic conditions the haloform reaction takes place and chloral hydrate is decomposed by hydrolysis to form chloroform.
[20] Chloral hydrate is metabolized in vivo to trichloroethanol, which is responsible for secondary physiological and psychological effects.
[21] The metabolite of chloral hydrate exerts its pharmacological properties via enhancing the GABA receptor complex[22] and therefore is similar in action to benzodiazepines, nonbenzodiazepines and barbiturates.
[23] Chloral hydrate is structurally and somewhat pharmacodynamically similar to ethchlorvynol, a pharmaceutical developed during the 1950s that was marketed as both a sedative and a hypnotic under the trade name Placidyl.
[25] In the United States, chloral hydrate is a schedule IV controlled substance and requires a physician's prescription.
[citation needed] Chloral hydrate was first synthesized by the chemist Justus von Liebig in 1832 at the University of Giessen.
[28][29][30] Its sedative properties were observed by Rudolf Buchheim in 1861, but described in detail and published only in 1869 by Oscar Liebreich;[31] subsequently, because of its easy synthesis, its use became widespread.
In 1869, German physician and pharmacologist Oscar Liebreich began to promote its use to calm anxiety, especially when it caused insomnia.
[34][33] Chloral hydrate had certain advantages over morphine for this application, as it worked quickly without injection and had a consistent strength.
This was not only the first attempt to determine whether different drugs were converted to the same metabolite in the body but also the first to measure the concentration of a particular pharmaceutical in the blood.
[36] In 1899 and 1901 Hans Horst Meyer and Ernest Overton respectively made the major discovery that the general anaesthetic action of a drug was strongly correlated to its lipid solubility.
A solution of chloral hydrate in ethanol called "knockout drops" was used to prepare a Mickey Finn.
[39] In 1897, Bram Stoker's epistolary novel Dracula, one of its characters, Doctor John Seward, recorded his use and his molecular formula in his phonographic diary: I cannot but think of Lucy, and how different things might have been.
[40]In the conclusion of Edith Wharton's 1905 novel The House of Mirth, Lily Bart, the novel's heroine, becomes addicted to chloral hydrate and overdoses on the substance: She put out her hand and measured the soothing drops into a glass; but as she did so, she knew they would be powerless against the supernatural lucidity of her brain.
[41]In the James Bond films From Russia With Love and The Living Daylights, chloral hydrate is used as a knockout drug.