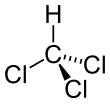
Chloroform
It is a volatile, colorless, sweet-smelling, dense liquid produced on a large scale as a precursor to refrigerants and PTFE.
[17] Chloroform was synthesized independently by several investigators c. 1831: In 1834, French chemist Jean-Baptiste Dumas determined chloroform's empirical formula and named it:[26] "Es scheint mir also erweisen, dass die von mir analysirte Substanz, … zur Formel hat: C2H2Cl6."
... "Diess hat mich veranlasst diese Substanz mit dem Namen 'Chloroform' zu belegen."
[11] At 400–500 °C, free radical halogenation occurs, converting these precursors to progressively more chlorinated compounds: Chloroform undergoes further chlorination to yield carbon tetrachloride (CCl4): The output of this process is a mixture of the four chloromethanes: chloromethane, methylene chloride (dichloromethane), trichloromethane (chloroform), and tetrachloromethane (carbon tetrachloride).
Sodium hypochlorite solution (chlorine bleach) mixed with common household liquids such as acetone, methyl ethyl ketone, ethanol, or isopropyl alcohol can produce some chloroform, in addition to other compounds, such as chloroacetone or dichloroacetone.
[citation needed] Chloroform is a powerful general anesthetic, euphoriant, anxiolytic, and sedative when inhaled or ingested.
The anaesthetic qualities of chloroform were first described in 1842 in a thesis by Robert Mortimer Glover, which won the Gold Medal of the Harveian Society for that year.
[28] A few days later, during the course of a dental procedure in Edinburgh, Francis Brodie Imlach became the first person to use chloroform on a patient in a clinical context.
[52] In May 1848, Robert Halliday Gunning made a presentation to the Medico-Chirurgical Society of Edinburgh following a series of laboratory experiments on rabbits that confirmed Glover's findings and also refuted Simpson's claims of originality.
[54] In the United States, chloroform began to replace ether as an anesthetic at the beginning of the 20th century;[citation needed] it was abandoned in favor of ether on discovery of its toxicity, especially its tendency to cause fatal cardiac arrhythmias analogous to what is now termed "sudden sniffer's death".
[57] Her autopsy establishing the cause of death was undertaken by John Fife assisted by Robert Mortimer Glover.
[citation needed] At the same time in the UK the medical journal The Lancet carried out a questionnaire survey[59] and compiled a report detailing numerous adverse reactions to anesthetics, including chloroform.
[citation needed] The rise of gas anaesthesia using nitrous oxide, improved equipment for administering anesthetics, and the discovery of hexobarbital in 1932 led to the gradual decline of chloroform narcosis.
[66] The use of chloroform as an incapacitating agent has become widely recognized, bordering on cliché, through the adoption by crime fiction authors of plots involving criminals' use of chloroform-soaked rags to render victims unconscious.
After a person has lost consciousness owing to chloroform inhalation, a continuous volume must be administered, and the chin must be supported to keep the tongue from obstructing the airway, a difficult procedure, typically requiring the skills of an anesthesiologist.
Reported ranges vary considerably, but are generally below the current health standard for total trihalomethanes (THMs) of 100 μg/L.
[69] However, when considered in combination with other trihalomethanes often present in drinking water, the concentration of THMs often exceeds the recommended limit of exposure.
[70] While few studies have assessed the risks posed by chloroform exposure through drinking water in isolation from other THMs, many studies have shown that exposure to the general category of THMs, including chloroform, is associated with an increased risk of cancer of the bladder or lower GI tract.
[71] Historically, chloroform exposure may well have been higher, owing to its common use as an anesthetic, as an ingredient in cough syrups, and as a constituent of tobacco smoke, where DDT had previously been used as a fumigant.
[38] Chloroform is metabolized in the liver by the cytochrome P-450 enzymes, by oxidation to trichloromethanol and by reduction to the dichloromethyl free radical.
Other metabolites of chloroform include hydrochloric acid and diglutathionyl dithiocarbonate, with carbon dioxide as the predominant end-product of metabolism.
Following chloroform-induced anesthesia, some patients suffered nausea, vomiting, hyperthermia, jaundice, and coma owing to hepatic dysfunction.
[73] Chloroform converts slowly in the presence of UV light and air to the extremely poisonous gas phosgene (COCl2), releasing HCl in the process.
Amylene has been found to be ineffective, and the phosgene can affect analytes in samples, lipids, and nucleic acids dissolved in or extracted with chloroform.
[81] It is classified as an extremely hazardous substance in the United States, as defined in Section 302 of the US Emergency Planning and Community Right-to-Know Act (42 U.S.C.