Chondroitin sulfate
[5] Chondroitin, along with commonly used glucosamine, should not be used to treat people who have symptomatic osteoarthritis of the knee as evidence shows that these treatments fail to provide relief for that condition.
[9][10] Clinical studies have not identified any significant side effects or overdoses of chondroitin sulfate, which suggest its long-term safety.
[11] In 2003 the Task Force of the European League Against Rheumatism (EULAR) committee ranked the level of toxicity of chondroitin sulfate 6 in a 0–100 scale.
[13] The effect of chondroitin sulfate in people with osteoarthritis is likely the result of a number of reactions including its anti-inflammatory activity, the stimulation of the synthesis of proteoglycans and hyaluronic acid, and the decrease in catabolic activity of chondrocytes, inhibiting the synthesis of proteolytic enzymes, nitric oxide, and other substances that contribute to damage the cartilage matrix and cause death of articular chondrocytes.
A recent review summarizes data from relevant reports describing the biochemical basis of the effect of chondroitin sulfate on osteoarthritis articular tissues.
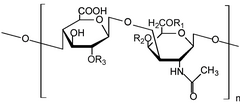
Chondroitin sulfate chains are unbranched polysaccharides of variable length containing two alternating monosaccharides: D-glucuronic acid (GlcA) and N-acetyl-D-galactosamine (GalNAc).
[27] In 2008 the U.S. Food and Drug Administration (FDA) identified "oversulfated chondroitin sulfate" as a contaminant in heparin originating from China.
[28][29][30] In 2004, a petition was submitted to the FDA that a dietary supplement of chondroitin sulfate be labeled as reducing the risk of osteoarthritis, cartilage deterioration, and osteoarthritis-related joint pain, tenderness, and swelling.
[5] Sawitzke A, et al. 2010 evaluated the efficacy and safety of glucosamine and chondroitin sulfate, alone or in combination, as well as celecoxib and placebo on painful knee osteoarthritis over 2 years as a continuation of the GAIT trial.
This was a 24-month, double-blind, placebo-controlled study, enrolling 662 people with knee osteoarthritis who satisfied radiographic criteria (Kellgren/Lawrence grade 2 or 3 changes and baseline joint space width of at least 2 mm).
The primary outcome was a 20% reduction in pain over 24 months as measured by the Western Ontario and McMaster University Osteoarthritis Index (WOMAC).
[5][33] Over 2 years, none of the treatments (not even the positive control celecoxib) achieved a clinically important difference in WOMAC pain or function as compared with placebo.
In Europe, chondroitin sulfate formulations are approved as drugs with evidenced efficacy and safety demonstrated by clinical trials in people with osteoarthritis.
[38] With the introduction of GMP regulations for dietary supplements in 2008, chondroitin sulfate preparations are subject in the US to mandatory labeling standards as well as testing requirements for identity, purity, strength, and composition.
[31] However, a proposed application of chondroitin sulfate dietary supplement as a means of preventing joint degeneration was highly scrutinized by the FDA, who stated: " For conventional foods, this evaluation involves considering whether the ingredient that is the source of the substance is generally recognized as safe (GRAS), approved as a food additive, or authorized by a prior sanction issued by FDA (see 21 CFR 101.70(f)).
They further denied the request to market it as safe, given that no human clinical trials were done, citing that animal studies are not sufficient for the approval of a dietary supplement.