Chytridiomycosis
In Australia, Panama, and New Zealand, the fungus seemed to have suddenly 'appeared' and expanded its range at the same time frog numbers declined.
[6] However, it may simply be that the fungus occurs naturally and was only identified recently because it has become more virulent or more prevalent in the environment, or because host populations have become less resistant to the disease.
[7] Lately, the genomes of 234 Batrachochytrium dendrobatidis isolates were phylogenetically compared and the results strongly suggest that a lineage found in the Korean peninsula likely seeded the panzootic.
[9][10] A later instance of a Bd-infected amphibian was a specimen of an African clawed frog (Xenopus laevis) collected in 1938, and this species also appears to be essentially unaffected by the disease, making it a suitable vector.
[11] If Batrachochytrium originated in Africa, the African clawed frog is thought to have been the vector of the initial spread out of the continent.
[6] When most species reach a B. dendrobatidis threshold of 10,000 zoospores, they are not able to breathe, hydrate, osmoregulate, or thermoregulate correctly.
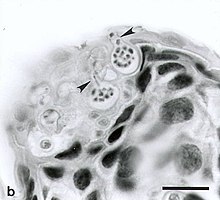
[17] The B. dendrobatidis' lifecycle continues until new zoospores are produced from the zoosporangium and exit to the environment or reinfect the same host.
[21] The limited range of B. dendrobatidis zoospores suggest some unknown mechanism exists by which they transmit from one host to the next,[21] which can involve the pet trade, and especially the American bullfrog.
[24] Besides amphibians Chytridiomycosis also infects crayfish (Procambarus alleni, P. clarkii, Orconectes virilis, and O. immunis) but not mosquitofish (Gambusia holbrooki).
[20] Individuals infected are also commonly found in a lethargic state, characterized by slow movements, and refuse to move when stimulated.
[20] A meta-analysis showed skin disruption, hormonal changes, and osmoregulation can occur with light infection, while higher pathogen loads are required to influence reproduction.
[28] In tadpoles, B. dendrobatidis affects the mouthparts, where keratin is present, leading to abnormal feeding behaviors or discoloration of the mouth.
[32] Although many declines have been credited to the fungus B. dendrobatidis—although likely prematurely so in many cases[4]—some species resist the infection and some populations can survive with a low level of persistence of the disease.
[34] Conservation efforts in New Zealand continue to be focused on curing the critically endangered native Archey's frog, Leiopelma archeyi, of chytridiomycosis, though research has shown clearly that they are immune from infection by B. dendrobatidis and are dying in the wild of other still-to-be identified diseases.
Due to the fungus' immense impact on amphibian populations, considerable research has been undertaken to devise methods to combat its proliferation in the wild.
Among the most promising is the revelation that amphibians in colonies that survive the passage of the chytrid epidemic tend to carry higher levels of the bacterium Janthinobacterium lividum.
[40] This bacterium produces antifungal compounds, such as indole-3-carboxaldehyde and violacein, that inhibit the growth of B. dendrobatidis even at low concentrations.
[41] Similarly, the bacterium Lysobacter gummosus found on the red-backed salamander (Plethodon cinereus), produces the compound 2,4-diacetylphloroglucinol that is inhibitory to the growth of B. dendrobatidis.
[44] Interactions between cutaneous microbiota and B. dendrobatidis can be altered to favor the resistance of the disease, as seen in past studies concerning the addition of the violacein-producing bacteria J. lividum to amphibians that lacked sufficient violacein, allowing them to inhibit infection.
[44][46] This implies that the antifungal bacterium J. lividum (native to other amphibians' skin, such as Hemidactylium scutatum) is able to produce a sufficient amount of violacein to prevent infection by B. dendrobatidis and allow coexistence with the potentially deadly fungus.
In particular, the skin peptide defenses were significantly reduced after exposure to carbaryl, suggesting pesticides may inhibit this innate immune defence, and increase susceptibility to disease.
[53] Rebound of frog species in Panama after decline are not associated with pathogen attenuation,[54][55] but rather a host factor - whether an evolved genetic resistance to the fungus infection, or an otherwise acquired trait (such as a hypothetically protective microbial colonization) is yet to be identified.
[citation needed] A study done by Rollins-Smith and colleagues suggests that itraconazole is the antifungal of choice when it comes to treatment of Bd.
[61] Experiments, where the temperature is increased beyond the upper bound of the B. dendrobatidis optimal range of 25 to 30 °C, show its presence will dissipate within a few weeks and infected individuals return to normal.
The amphibian host and even the environment can be augmented with probiotic bacteria that express anti-fungal metabolites that can fight B. dendrobatidis.