Coniine
Coniine is a poisonous chemical compound, an alkaloid present in and isolable from poison hemlock (Conium maculatum), where its presence has been a source of significant economic, medical, and historico-cultural interest; coniine is also produced by the yellow pitcher plant (Sarracenia flava), and fool's parsley (Aethusa cynapium).
Its ingestion and extended exposure are toxic to humans and all classes of livestock; its mechanism of poisoning involves disruption of the central nervous system, with death caused by respiratory paralysis.
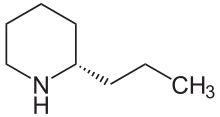
This pathway results in natural coniine that is a mixture—a racemate—composed of two enantiomers, the stereoisomers (S)-(+)-coniine and (R)-(−)-coniine, depending on the direction taken by the chain that branches from the ring.
Its presence on farmland is an issue for livestock farmers because animals will eat it if they are not well fed or the hemlock is mixed in with pasture grass.
The plant uses a mixture of sugar and coniine to simultaneously attract and poison insects, which then fall into a digestive tube.
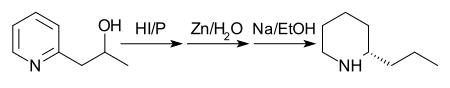
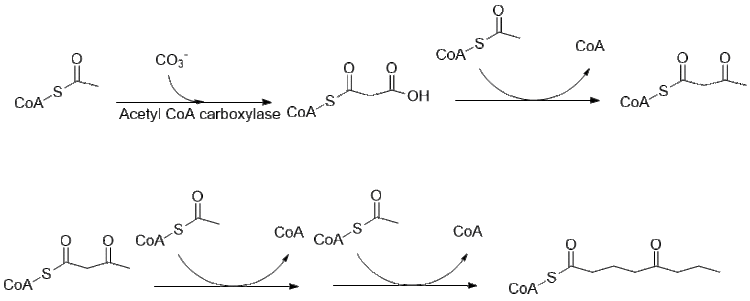
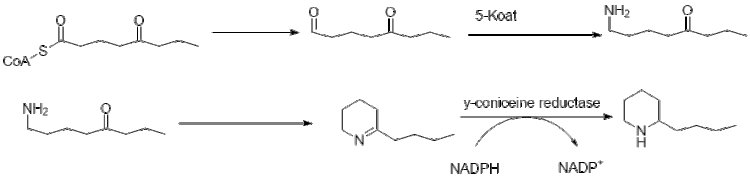
[6] The history of coniine is understandably tied to the poison hemlock plant, since the natural product was not synthesizable until the 1880s.
[7] Jews in the Middle East were poisoned by coniine after consuming quail in the area that usually ate hemlock seeds, and Greeks on the island of Lesbos who also consumed quail suffered from the same poisoning, causing myoglobinuria and acute kidney injury.
[13] In the Middle Ages it was believed that hemlock could be used to cure rabies; in later European times it came to be associated with flying ointments in witchcraft.
[2] Coniine, as racemate or as pure enantiomer, begins by binding and stimulating the nicotinic receptor on the post-synaptic membrane of the neuromuscular junction.
The central nervous system is not affected: the person remains conscious and aware until respiratory paralysis results in cessation of breathing.
Cause of death is lack of oxygen to the brain and heart as a consequence of respiratory paralysis, so that a poisoned person may recover if artificial ventilation can be maintained until the toxin is removed from the victim's system.
[20][21] D-(S)-Coniine has since been determined to be a colorless alkaline liquid, with a penetrating odour and a burning taste; has D0° 0.8626 and D19° 0.8438, refractive index n23°D 1.4505, and is dextrorotatory, [α]19°D +15.7° (see related comments under Specific rotation section below).
L-(R)-Coniine has [α]21°D 15° and in other respects resembles its D-isomer, but the salts have slightly different melting points; the platinichloride has mp.
Sodium nitroprusside gives a deep red color, which disappears on warming, but reappears on cooling, and is changed to blue or violet by aldehydes.
[27] The stereochemical composition of "coniine" is a matter of some importance, since its two enantiomers do not have identical biological properties,[2] and many of the older pharmacological studies on this compound were carried out using the naturally-occurring isomeric mixture.
Note: although the scheme below shows a single enantiomer of coniine, the final reaction produces a racemic mixture that is then separated In 1907, another route with better yield was proposed.
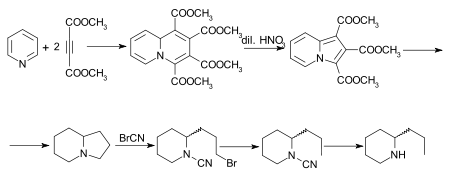
[33] Note: although the graphic below shows a single enantiomer of coniine, this reaction produces a racemic mixture that is then purified and separated.
[35] The initial adduct of pyridine and dimethyl acetylenedicarboxylate is tetramethylquinolizine-1,2,3,4-tetracarboxylate, which on oxidation with dilute nitric acid is converted into trimethyl indolizine-tricarboxylate.
This, on hydrolysis and decarboxylation, furnishes indolizine, the octahydro-derivate of which, also known as octahydropyrrocoline[36] is converted by the cyanogen bromide method successively into the bromocyanamide, cyanamide and rac.-coniine.
[38] Hess and Eichel reported,[39] incorrectly,[40] that pelletierine was the aldehyde (β-2-piperidyl-propaldehyde) corresponding to coniine, and yielded rac-coniine when its hydrazone was heated with sodium ethoxide in ethanol at 156–170 °C.
[31] For example, Pd-catalyzed 1,3-chirality transfer reaction can stereospecifically transform a single enantiomer of an allyl alcohol into a cyclic structure (in this case a piperidine).