Copper(II) nitrate
Copper(II) nitrate describes any member of the family of inorganic compounds with the formula Cu(NO3)2(H2O)x.
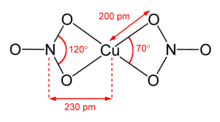
Such complexes are highly labile and subject to rapid ligand exchange due to the d9 electronic configuration of copper(II).
Attempted dehydration of any of the hydrated copper(II) nitrates by heating affords the oxides, not Cu(NO3)2.
[7] Exploiting this reactivity, copper nitrate can be used to generate nitric acid by heating it until decomposition and passing the fumes directly into water.
[6] Both polymorphs are three-dimensional coordination polymer networks with infinite chains of copper(II) centers and nitrate groups.
[16] The crystal structure of the hexahydrate appeared to show six almost equal Cu–O distances, not revealing the usual effect of a Jahn-Teller distortion that is otherwise characteristic of octahedral Cu(II) complexes.
[17] Hydrated copper nitrate adsorbed onto clay affords a reagent called "Claycop".
[21][22] Natural basic copper nitrates include the rare minerals gerhardtite and rouaite, both being polymorphs of Cu2(NO3)(OH)3.