Cubic crystal system
There are three main varieties of these crystals: Note: the term fcc is often used in synonym for the cubic close-packed or ccp structure occurring in metals.
(Loosely packed arrangements do occur, though, for example if the orbital hybridization demands certain bond angles.)
Accordingly, the primitive cubic structure, with especially low atomic packing factor, is rare in nature, but is found in polonium.
Unlike fcc and bcc, this structure is not a lattice, since it contains multiple atoms in its primitive cell.
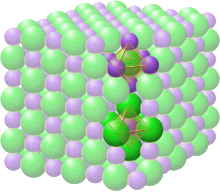
However, the caesium chloride structure has a basis composed of two different atomic species.
In addition to caesium chloride itself, the structure also appears in certain other alkali halides when prepared at low temperatures or high pressures.
The space group of the caesium chloride (CsCl) structure is called Pm3m (in Hermann–Mauguin notation), or "221" (in the International Tables for Crystallography).
Other compounds showing caesium chloride like structure are CsBr, CsI, high-temperature RbCl, AlCo, AgZn, BeCu, MgCe, RuAl and SrTl.
[citation needed] The space group of the rock-salt or halite (sodium chloride) structure is denoted as Fm3m (in Hermann–Mauguin notation), or "225" (in the International Tables for Crystallography).
The structure can also be described as an FCC lattice of sodium with chlorine occupying each octahedral void or vice versa.
[6] Examples of compounds with this structure include sodium chloride itself, along with almost all other alkali halides, and "many divalent metal oxides, sulfides, selenides, and tellurides".
Many transition metal monoxides also have the rock salt structure (TiO, VO, CrO, MnO, FeO, CoO, NiO, CdO).
The early actinoid monocarbides also have this structure (ThC, PaC, UC, NpC, PuC).
As in the rock-salt structure, the two atom types form two interpenetrating face-centered cubic lattices.
The structure can also be described as an FCC lattice of zinc with sulfur atoms occupying half of the tetrahedral voids or vice versa.
[53][54] This group is also known as the II-VI family of compounds, most of which can be made in both the zincblende (cubic) or wurtzite (hexagonal) form.
Examples occur among the transition metal silicides and germanides, as well as a few other compounds such as gallium palladide.
Gas hydrates formed by methane, propane, and carbon dioxide at low temperatures have a structure in which water molecules lie at the nodes of the Weaire–Phelan structure and are hydrogen bonded together, and the larger gas molecules are trapped in the polyhedral cages.