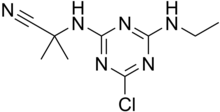
Cyanazine
Cyanazine and the other triazines have been among the group of most heavily used herbicides in the mid-west and the United States of America.
[clarification needed] Cyanazine is not very reactive in neutral and slightly acidic/basic media, it is hydrolysed by strong acids and bases.
[8] Cyanazine is the most toxic triazine herbicide and can cause birth defects, mutations and ultimately cancer.
Different studies showed that in animal models (rats, dogs & cows) the cyanazine is quickly absorbed in the intestines.
For the degradation of the absorbed cyanazine the following metabolic pathways are involved: de-alkylation & conjugation with glutathione, which results in different metabolites.
Another major route of degradation for cyanazine in mammals is N-De-ethylation, which leads to the yield of an ethyl group.
[11][12] In plants the following metabolic pathways are involved hydrolysis, N-de-alkylation and conjugation with glutathione, resulting in different metabolites (shown in the figure below).
[13] Cyanazine is used as a herbicide to control annual grasses and broadleaf weeds in corn, grain, sorghum, cotton and wheat fallow.
[15] In animals and algae, the LD50/LC50/EC50 are as following:[16] After repeated doses of 25 ppm cyanazine mixed in rat diets, no toxic effects were seen.
Cyanazine will result in the dysfunction of photosystem II by binding important proteins which are required for this process.
When this important step in photosynthesis fails, a plant is not able to produce sugars which are crucial for its growth and metabolism.
In Silurana tropicalis, exposure to triazine herbicides like cyanazine may cause severe abnormalities.
In the case of cyanazine, atrazine can cause effects on non-target species like Chironomus tentans.
Atrazine is able to influence the activity of P450 enzymes in midges and therefore cause increased toxicity of these herbicides.