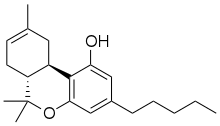
Δ-8-Tetrahydrocannabinol
After the 2018 United States farm bill was signed, ∆8-THC products partially synthesized from industrial hemp experienced a rise in popularity; THC products have been sold in licensed recreational cannabis and medical cannabis industries within the United States in California, Pennsylvania, and medicinally licensed in Michigan and Oregon.
[6] Both isomers of THC have been found to cause a transient increase in blood pressure in rats,[7] although the effects of cannabinoids on the cardiovascular system are complex.
[8] Animal studies indicate that ∆8-THC exerts many of its central effects by binding to cannabinoid receptors found in various regions of the brain, including the cerebral cortex, thalamus, basal ganglia, hippocampus, and cerebellum.
This difference in structure increases the chemical stability of ∆8-THC relative to ∆9-THC, lengthening shelf life and allowing the compound to resist undergoing oxidation to cannabinol over time.
[24] While ∆8-THC is naturally found in plants of the Cannabis genus,[1] this compound can also be produced in an industrial or laboratory setting by acid-catalyzed isomerization of cannabidiol.
[34] In 1942, the same research group studied its physiological and psychoactive effects after oral dosing in human volunteers.
[36] In 1966, the chemical structure of ∆8-THC isolated from cannabis was characterized using modern methods by Richard L. Hively, William A. Mosher, and Friedrich W. Hoffmann at the University of Delaware.
[37] A stereospecific synthesis of ∆8-THC from olivetol and verbenol was reported by Raphael Mechoulam and colleagues at the Weizmann Institute of Science in 1967.
[44] Under section 297A of that subtitle, is the definition of hemp as used in federal law: The term "hemp" means the plant Cannabis sativa L. and any part of that plant, including the seeds thereof and all derivatives, extracts, cannabinoids, isomers, acids, salts, and salts of isomers, whether growing or not, with a delta-9 tetrahydrocannabinol concentration of not more than 0.3 percent on a dry weight basis.In October 2020, the DEA Interim Final Rule[45] addressed synthetic cannabinoids.
[46] The University of Arkansas National Agriculture Law Center has maintained an index of litigation surrounding hemp and delta-8 products.
[50] The FDA has also taken action against businesses that sold ∆8-THC in forms that closely resemble (typically non-psychoactive) food products such as chips or cookies.
[51][52][53][54] In 2021, one store owner in Menomonee Falls, Wisconsin was facing a sentence of up to 50 years for allegedly selling ∆8-THC products with illegal amounts of ∆9-THC.
[55] Other raids and arrests have happened due to the ∆9-THC content of these products in North Carolina, and Texas, among other places.
"[59] There are also issues related to incidental manufacture of ∆9 THC, as ∆9 is produced as an intermediate product in the process of acid catalyzed ring closure of cannabidiol.
[64] The Fourth Circuit Court of Appeals has upheld ∆8-THC regulations in Virginia, also finding the Farm Bill did not preempt state law.
[65] Common Delta-8 products range from bulk quantities of unrefined distillate to prepared cannabis edibles and atomizer cartridges.
[68] ∆8-THC products partially synthesized from industrial hemp experienced a rise in popularity in the US following the passage of the 2018 Farm Bill.
[70][71] In March 2024, a study of self-reported prevalence of Δ8-THC use among US twelfth graders was published: Of those reporting Δ8-THC use, 35% had used it at least 10 times in the past 12 months.