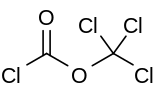
Diphosgene
This colorless liquid is a valuable reagent in the synthesis of organic compounds.
Diphosgene is prepared by radical chlorination of methyl chloroformate under UV light:[1] Another method is the radical chlorination of methyl formate:[2] Diphosgene converts to phosgene upon heating or upon catalysis with charcoal.
Diphosgene was originally developed as a pulmonary agent for chemical warfare, a few months after the first use of phosgene.
[4] Diphosgene was developed because the vapors could destroy the filters of the gas masks in use at the time.
Diphosgene has a relatively high vapor pressure of 10 mm Hg (1.3 kPa) at 20 °C and decomposes to phosgene around 300 °C.