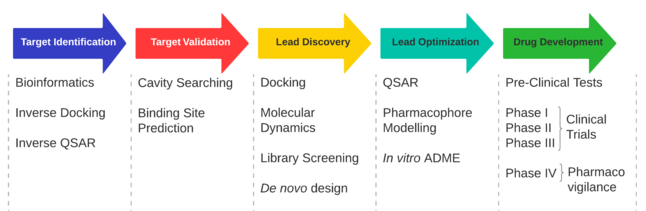
Drug discovery
[1] Historically, drugs were discovered by identifying the active ingredient from traditional remedies or by serendipitous discovery, as with penicillin.
More recently, chemical libraries of synthetic small molecules, natural products, or extracts were screened in intact cells or whole organisms to identify substances that had a desirable therapeutic effect in a process known as classical pharmacology.
[5] Modern drug discovery is thus usually a capital-intensive process that involves large investments by pharmaceutical industry corporations as well as national governments (who provide grants and loan guarantees).
This made for the beginning of the modern era in pharmacology, as pure chemicals, instead of crude extracts of medicinal plants, became the standard drugs.
Examples of drug compounds isolated from crude preparations are morphine, the active agent in opium, and digoxin, a heart stimulant originating from Digitalis lanata.
[11] Later, small molecules were synthesized to specifically target a known physiological/pathological pathway, avoiding the mass screening of banks of stored compounds.
[15] Another champion of the approach of developing chemical analogues of known active substances was Sir David Jack at Allen and Hanbury's, later Glaxo, who pioneered the first inhaled selective beta2-adrenergic agonist for asthma, the first inhaled steroid for asthma, ranitidine as a successor to cimetidine, and supported the development of the triptans.
[16] Gertrude Elion, working mostly with a group of fewer than 50 people on purine analogues, contributed to the discovery of the first anti-viral; the first immunosuppressant (azathioprine) that allowed human organ transplantation; the first drug to induce remission of childhood leukemia; pivotal anti-cancer treatments; an anti-malarial; an anti-bacterial; and a treatment for gout.
[8] Generally, the "target" is the naturally existing cellular or molecular structure involved in the pathology of interest where the drug-in-development is meant to act.
At this point, medicinal chemists will attempt to use structure–activity relationships (SAR) to improve certain features of the lead compound: This process will require several iterative screening runs, during which, it is hoped, the properties of the new molecular entities will improve, and allow the favoured compounds to go forward to in vitro and in vivo testing for activity in the disease model of choice.
Amongst the physicochemical properties associated with drug absorption include ionization (pKa), and solubility; permeability can be determined by PAMPA and Caco-2.
For example, virtual screening and computer-aided drug design are often used to identify new chemical moieties that may interact with a target protein.
[44][45][46] The advantages of these approaches are that they allow more efficient screening and the compound library, although small, typically covers a large chemical space when compared to HTS.
[49] In many cases, the exact mechanism of action of hits from these screens is unknown and may require extensive target deconvolution experiments to ascertain.
[citation needed] Natural products may be useful as a source of novel chemical structures for modern techniques of development of antibacterial therapies.
[63] This has resulted in a pool of information about the potential of plant species as important sources of starting materials for drug discovery.
They induce apoptosis[67][68] and protein cascade via proteinase inhibitor,[67] have defense functions,[69] and regulate plant responses to different biotic and abiotic stresses.
[73] The discovery of JADs on skin repair has introduced newfound interest in the effects of these plant hormones in therapeutic medicinal application.
For example, the anticoagulant drugs, hirudin and its synthetic congener, bivalirudin, are based on saliva chemistry of the leech, Hirudo medicinalis.
[76] Used to treat type 2 diabetes, exenatide was developed from saliva compounds of the Gila monster, a venomous lizard.
The classic example of an antibiotic discovered as a defense mechanism against another microbe is penicillin in bacterial cultures contaminated by Penicillium fungi in 1928.
[citation needed] [dubious – discuss] For example, the cone snail toxin ziconotide, also known as Prialt treats severe neuropathic pain.
[citation needed] Two main approaches exist for the finding of new bioactive chemical entities from natural sources.
[citation needed] A collection of plant, animal and microbial samples from rich ecosystems can potentially give rise to novel biological activities worth exploiting in the drug development process.
Paclitaxel showed anti-tumour activity by a previously undescribed mechanism (stabilization of microtubules) and is now approved for clinical use for the treatment of lung, breast, and ovarian cancer, as well as for Kaposi's sarcoma.
Artemisinin, an antimalarial agent from sweet wormtree Artemisia annua, used in Chinese medicine since 200BC is one drug used as part of combination therapy for multiresistant Plasmodium falciparum.
By using machine learning algorithms to analyse large amounts of chemical data, researchers can identify potential new drug candidates that are more likely to be effective against a specific disease.
Algorithms, such as Nearest-Neighbour classifiers, RF, extreme learning machines, SVMs, and deep neural networks (DNNs), are used for VS based on synthesis feasibility and can also predict in vivo activity and toxicity.
Nuclear magnetic resonance spectroscopy is the primary technique for determining chemical structures of natural products.
NMR yields information about individual hydrogen and carbon atoms in the structure, allowing detailed reconstruction of the molecule's architecture.