Pharmaceutical industry
[3] In historical terms, the pharmaceutical industry, as an intellectual concept, arose in the middle to late 1800s in nation-states with developed economies such as Germany, Switzerland, and the United States.
Some businesses engaging in synthetic organic chemistry, such as several firms generating dyestuffs derived from coal tar on a large scale, were seeking out new applications for their artificial materials in terms of human health.
This trend of increased capital investment occurred in tandem with the scholarly study of pathology as a field advancing significantly, and a variety of businesses set up cooperative relationships with academic laboratories evaluating human injury and disease.
[4] The modern era of the pharmaceutical industry began with local apothecaries that expanded their traditional role of distributing botanical drugs such as morphine and quinine to wholesale manufacture in the mid-1800s.
[citation needed] By the 1890s, the profound effect of adrenal extracts on many different tissue types had been discovered, setting off a search both for the mechanism of chemical signaling and efforts to exploit these observations for the development of new drugs.
A structurally similar compound, ephedrine, was identified by Japanese chemists in the Ma Huang plant and marketed by Eli Lilly as an oral treatment for asthma.
In 1921, Canadian professor Frederick Banting and his student Charles Best repeated this study and found that injections of pancreatic extract reversed the symptoms produced by pancreas removal.
Soon, the extract was demonstrated to work in people, but the development of insulin therapy as a routine medical procedure was delayed by difficulties in producing the material in sufficient quantity and with reproducible purity.
Chemist George B. Walden of Eli Lilly and Company found that careful adjustment of the pH of the extract allowed a relatively pure grade of insulin to be produced.
Under pressure from Toronto University and a potential patent challenge by academic scientists who had independently developed a similar purification method, an agreement was reached for the non-exclusive production of insulin by multiple companies.
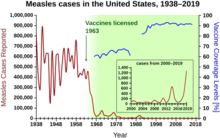
[22] There was early progress toward the development of vaccines throughout this period, primarily in the form of academic and government-funded basic research directed toward the identification of the pathogens responsible for common communicable diseases.
In 1923, parallel efforts by Gaston Ramon at the Pasteur Institute and Alexander Glenny at the Wellcome Research Laboratories (later part of GlaxoSmithKline) led to the discovery that a safer vaccine could be produced by treating diphtheria toxin with formaldehyde.
The study further examined mortality rates for eight common diseases for which antibiotics offered effective therapy (syphilis, tuberculosis, dysentery, scarlet fever, whooping cough, meningococcal infections, and pneumonia), and found a 56% decline over the same period.
[56] Early developments in the field of treating hypertension included quaternary ammonium ion sympathetic nervous system blocking agents, but these compounds were never widely used due to their severe side effects, because the long-term health consequences of high blood pressure had not yet been established, and because they had to be administered by injection.
In the mid-1950s Karl H. Beyer, James M. Sprague, John E. Baer, and Frederick C. Novello of Merck and Co. discovered and developed chlorothiazide, which remains the most widely used antihypertensive drug today.
The history of the development of oral contraceptives is thus closely tied to the birth control movement and the efforts of activists Margaret Sanger, Mary Dennett, and Emma Goldman.
While momentum for new legislation temporarily flagged under extended debate, a new tragedy emerged that underscored the need for more comprehensive regulation and provided the driving force for the passage of new laws.
[82] Often, large multinational corporations exhibit vertical integration, participating in a broad range of drug discovery and development, manufacturing and quality control, marketing, sales, and distribution.
In England and Wales NICE decides whether and in what circumstances drugs and technologies will be made available by the NHS, whilst similar arrangements exist with the Scottish Medicines Consortium in Scotland, and the Pharmaceutical Benefits Advisory Committee in Australia.
IMS Health publishes an analysis of trends expected in the pharmaceutical industry in 2007, including increasing profits in most sectors despite loss of some patents, and new 'blockbuster' drugs on the horizon.
Pharmaceutical companies generally employ salespeople (often called 'drug reps' or, an older term, 'detail men') to market directly and personally to physicians and other healthcare providers.
[112] In 2009, the Government-funded National Prescribing Service launched the "Finding Evidence – Recognising Hype" program, aimed at educating GPs on methods for independent drug analysis.
It has been argued that the design of the Diagnostic and Statistical Manual of Mental Disorders and the expansion of the criteria represents an increasing medicalization of human nature, or "disease mongering", driven by drug company influence on psychiatry.
[117] The potential for direct conflict of interest has been raised, partly because roughly half the authors who selected and defined the DSM-IV psychiatric disorders had or previously had financial relationships with the pharmaceutical industry.
[123] Others have argued that excessive regulation suppresses therapeutic innovation and that the current cost of regulator-required clinical trials prevents the full exploitation of new genetic and biological knowledge for the treatment of human disease.
Legal claims against the pharmaceutical industry have varied widely over the past two decades, including Medicare and Medicaid fraud, off-label promotion, and inadequate manufacturing practices.
[134][135] In May 2015, the New England Journal of Medicine emphasized the importance of pharmaceutical industry-physician interactions for the development of novel treatments and argued that moral outrage over industry malfeasance had unjustifiably led many to overemphasize the problems created by financial conflicts of interest.
This provision is intended to encourage the development of drugs affecting fewer than 200,000 Americans by granting strengthened and extended legal monopoly rights to the manufacturer, along with waivers on taxes and government fees.
[148][149] Remdesivir is a candidate for treating COVID-19; at the time the status was granted, fewer than 200,000 Americans had COVID-19, but numbers were climbing rapidly as the COVID-19 pandemic reached the US, and crossing the threshold soon was considered inevitable.
In 2001, the WTO adopted the Doha Declaration, which indicates that the TRIPS agreement should be read with the goals of public health in mind, and allows some methods for circumventing pharmaceutical monopolies: via compulsory licensing or parallel imports, even before patent expiration.