Elias James Corey
In 1990, he won the Nobel Prize in Chemistry "for his development of the theory and methodology of organic synthesis",[3] specifically retrosynthetic analysis.
[4][5] Regarded by many as one of the greatest living chemists, he has developed numerous synthetic reagents, methodologies and total syntheses and has advanced the science of organic synthesis considerably.
Corey (the surname was anglicized from Levantine Arabic Khoury, meaning priest) was born to Lebanese Greek Orthodox Christian immigrants Fatima (née Hasham) and Elias Corey in Methuen, Massachusetts, 50 km (31 mi) north of Boston.
[6] His mother changed his name from William to "Elias" to honor his father, who died eighteen months after Corey's birth.
His widowed mother, brother, two sisters, aunt and uncle all lived together in a spacious house, struggling through the Great Depression.
At the age of 16 Corey entered MIT, where he earned both a bachelor's degree in 1948 and a Ph.D. under Professor John C. Sheehan in 1951.
Upon entering MIT, Corey's only experience with science was in mathematics, and he began his college career pursuing a degree in engineering.
[7] In 1959, he moved to Harvard University, where he is currently an emeritus professor of organic chemistry with an active Corey Group research program.
Corey has developed several new synthetic reagents: In the reaction, the alcohol nucleophilically displaces chlorine from the electropositive chromium(VI) metal.
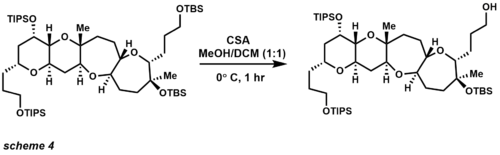
Acidic conditions usually accomplish cleavage of MEM protecting groups, but coordination with metal halides greatly enhances lability (scheme 6).
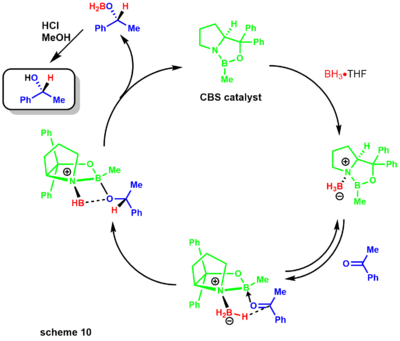
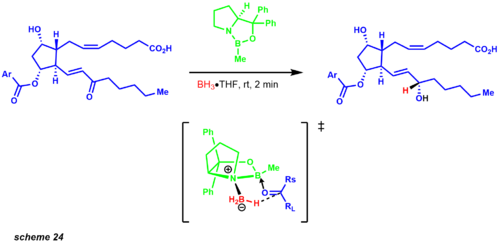
[31] Poor donation from the nitrogen to the boron leaves the Lewis acidity mostly intact, allowing coordination to the ketone substrate.
The complexation of the substrate occurs from the most accessible lone pair of the oxygen, restricting rotation around the B-O bond due to the sterically neighboring phenyl group.
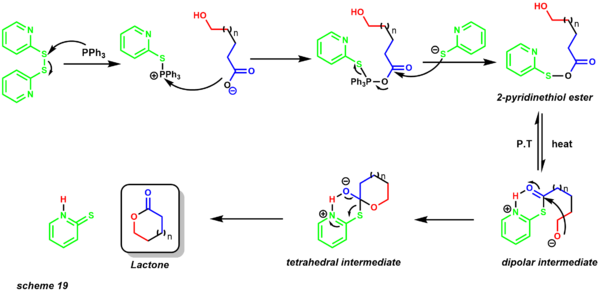
The reaction accommodates a wide array of functional groups, but allylic and benzylic alcohols are typically transformed into chlorides instead.
The synthesis of natural products using the Diels-Alder reaction as a transform has been applied especially to the formation of six-membered rings(scheme 18).
The presence of both cis and trans olefins as well as five asymmetric carbon atoms renders the molecule a desirable challenge for organic chemists.
Molecular simplification began first by disconnecting both carbon chains with a Wittig reaction and Horner-Wadsworth Emmons modification.
One of the examples that exemplified this protocol was an intermediate in the prostaglandin synthesis revealing a 9:1 mixture of the desired diastereomer (scheme 24).
The pivotal intermediate leads to a straightforward conversion to the Diels-Alder structural goal, which provides the carbon framework for the functionalized cyclopentane ring.
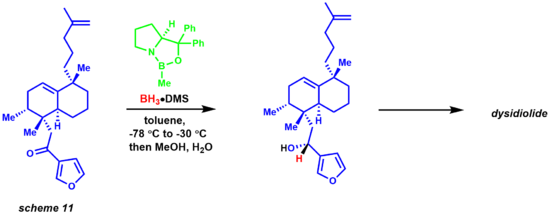
Later Corey developed an asymmetric Diels-Alder reaction employing a chiral oxazoborolidine, greatly simplifying the synthetic route to the prostaglandins.
Other notable syntheses: Corey and his research group created LHASA, a program that uses artificial intelligence to discover sequences of reaction which may lead to total synthesis.
[77] As of 2010, approximately 700 people have been Corey Group members including notable students Eric Block, Dale L. Boger, Weston T. Borden, David E. Cane, Rick L. Danheiser, William L. Jorgensen, John Katzenellenbogen, Alan P. Kozikowski, Bruce H. Lipshutz, David R. Liu, Albert Meyers, K. C. Nicolaou, Ryōji Noyori, Gary H. Posner, Bengt I. Samuelsson, Dieter Seebach, Vinod K. Singh, Brian Stoltz, Alice Ting, Hisashi Yamamoto, Phil Baran and Jin-Quan Yu.
[80] When awarded the Priestley Medal in 2004, E. J. Corey created a controversy with his claim to have inspired Robert Burns Woodward prior to the development of the Woodward–Hoffmann rules.
Corey wrote: "On May 4, 1964, I suggested to my colleague R. B. Woodward a simple explanation involving the symmetry of the perturbed (HOMO) molecular orbitals for the stereoselective cyclobutene → 1,3-butadiene and 1,3,5-hexatriene → cyclohexadiene conversions that provided the basis for the further development of these ideas into what became known as the Woodward–Hoffmann rules.
Corey also hoped that Woodward himself would correct the historical record "as he grew older, more considerate, and more sensitive to his own conscience.

![[3,3] rearrangement with PCC](http://upload.wikimedia.org/wikipedia/commons/thumb/8/87/PCC_rearrangement3.png/700px-PCC_rearrangement3.png)