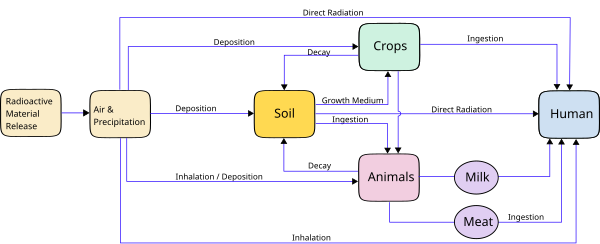
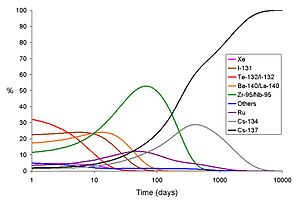
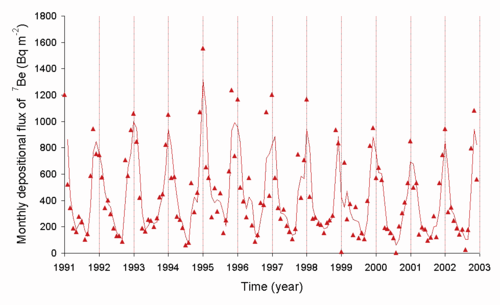
Environmental radioactivity
The concentration and location of some natural isotopes, particularly uranium-238 (238U), can be affected by human activity, such as nuclear weapons testing, which caused a global fallout, with up to 2.4 million deaths by 2020.
Some relationship between distance and activity can be seen in their data, when fitted to an exponential curve, but the scatter of the points is large (R2 = 0.3683).
Jiří Hála claims in his textbook "Radioactivity, Ionizing Radiation and Nuclear Energy" [6] that cattle only pass a minority of the strontium, caesium, plutonium and americium they ingest to the humans who consume milk and meat.
The glassy trinitite created by the first atom bomb contains radioisotopes formed by neutron activation and nuclear fission.
The 152Eu (half life 13.54 year) and 154Eu (half life 8.59 year) were mainly formed by the neutron activation of the europium in the soil, it is clear that the level of radioactivity for these isotopes is highest where the neutron dose to the soil was larger.
The 137Cs level is higher in the sample that was further away from the ground zero point – this is thought to be because the precursors to the 137Cs (137I and 137Xe) and, to a lesser degree, the caesium itself are volatile.
[citation needed] Nuclear bomb tests have increased the specific activity of carbon, whereas the use of fossil fuels has decreased it.
[citation needed] In addition, a zinc activation product (65Zn) was found, this is thought to be due to the corrosion of magnox fuel cladding in cooling ponds.
[8] The concentration of all these isotopes in the Irish Sea attributable to nuclear facilities such as Sellafield has significantly decreased in recent decades.
An important part of the Chernobyl release was the caesium-137, this isotope is responsible for much of the long term (at least one year after the fire) external exposure which has occurred at the site.
[2] A large amount of caesium was released during the Goiânia accident where a radioactive source (made for medical use) was stolen and then smashed open during an attempt to convert it into scrap metal.
This paper also reports details of the effect of potassium, ammonium and calcium ions on the uptake of the radioisotopes.
Caesium binds tightly to clay minerals such as illite and montmorillonite; hence it remains in the upper layers of soil where it can be accessed by plants with shallow roots (such as grass).
Also, after a nuclear war or serious accident, the removal of top few cm of soil and its burial in a shallow trench will reduce the long term gamma dose to humans due to 137Cs as the gamma photons will be attenuated by their passage through the soil.
The cyanide is so tightly bonded to the iron that it is safe for a human to eat several grams of prussian blue per day.
An added advantage of the prussian blue is that the caesium which is stripped from the animal in the droppings is in a form which is not available to plants.
By measuring cosmogenic isotopes, scientists are able to gain insight into a range of geological and astronomical processes.
Because cosmogenic isotopes have long half-lives (anywhere from thousands to millions of years), scientists find them useful for geologic dating.
Cosmogenic isotopes are produced at or near the surface of the Earth, and thus are commonly applied to problems of measuring ages and rates of geomorphic and sedimentary events and processes.
Zircon incorporates uranium atoms into its crystalline structure as substitutes for zirconium, but strongly rejects lead.
One of the advantages of this method is that any sample provides two clocks, one based on uranium-235's decay to lead-207 with a half-life of about 703 million years, and one based on uranium-238's decay to lead-206 with a half-life of about 4.5 billion years, providing a built-in crosscheck that allows accurate determination of the age of the sample even if some of the lead has been lost.