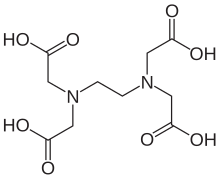
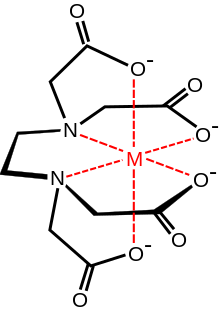
Ethylenediaminetetraacetic acid
In the pulp and paper industry, EDTA inhibits the ability of metal ions, especially Mn2+, from catalysing the disproportionation of hydrogen peroxide, which is used in chlorine-free bleaching.
In a similar manner, EDTA is added to some food as a preservative or stabiliser to prevent catalytic oxidative decolouration, which is catalysed by metal ions.
[5] The reduction of water hardness in laundry applications and the dissolution of scale in boilers both rely on EDTA and related complexants to bind Ca2+, Mg2+, as well as other metal ions.
Perfected by F. H. Spedding et al. in 1954, the method relies on the steady increase in stability constant of the lanthanide EDTA complexes with atomic number.
Due to the expense of this method, relative to countercurrent solvent extraction, ion exchange is now used only to obtain the highest purities of lanthanides (typically greater than 99.99%).
[citation needed] Sodium calcium edetate, an EDTA derivative, is used to bind metal ions in the practice of chelation therapy, such as for treating mercury and lead poisoning.
[12] EDTA is a slime dispersant, and has been found to be highly effective in reducing bacterial growth during implantation of intraocular lenses (IOLs).
[13] Dentists and endodontists use EDTA solutions to remove inorganic debris (smear layer) and lubricate the root canals in endodontics.
Some alternative practitioners believe EDTA acts as an antioxidant, preventing free radicals from injuring blood vessel walls, therefore reducing atherosclerosis.
[16] In shampoos, cleaners, and other personal care products, EDTA salts are used as a sequestering agent to improve their stability in air.
[18] EDTA also acts as a selective inhibitor against dNTP hydrolyzing enzymes (Taq polymerase, dUTPase, MutT),[19] liver arginase[20] and horseradish peroxidase[21] independently of metal ion chelation.
These findings urge the rethinking of the utilisation of EDTA as a biochemically inactive metal ion scavenger in enzymatic experiments.
In analytical chemistry, EDTA is used in complexometric titrations and analysis of water hardness or as a masking agent to sequester metal ions that would interfere with the analyses.
EDTA finds many specialised uses in the biomedical labs, such as in veterinary ophthalmology as an anticollagenase to prevent the worsening of corneal ulcers in animals.
However, it may influence the bioavailability of metals in solution, which may pose concerns regarding its effects in the environment, especially given its widespread uses and applications.
Impurities cogenerated by this route include glycine and nitrilotriacetic acid; they arise from reactions of the ammonia coproduct.
[34] Depending on the light conditions, the photolysis half-lives of iron(III) EDTA in surface waters can range as low as 11.3 minutes up to more than 100 hours.
Some microorganisms have even been discovered to form nitrates out of EDTA, but they function optimally at moderately alkaline conditions of pH 9.0–9.5.
In addition to having a lower toxicity after chelation, IDS is degraded by Agrobacterium tumefaciens (BY6), which can be harvested on a large scale.
[45] Preparation of hydrogels based on polyaspartic acid, in a variety of physical forms ranging from fiber to particle, can potentially enable facile separation of the chelated ions from a solution.
[47] A structural isomer of EDTA, ethylenediamine-N,N′-disuccinic acid (EDDS) is readily biodegradable at high rate in its S,S form.
[48] Trisodium dicarboxymethyl alaninate, also known as methylglycinediacetic acid (MGDA), has a high rate of biodegradation at over 68%, but unlike many other chelating agents can degrade without the assistance of adapted bacteria.
Additionally, unlike EDDS or IDS, MGDA can withstand higher temperatures while maintaining a high stability as well as the entire pH range.
[citation needed] MGDA has been shown to be an effective chelating agent, with a capacity for mobilization comparable with that of nitrilotriacetic acid (NTA), with application to water for industrial use and for the removal of calcium oxalate from urine from patients with kidney stones.