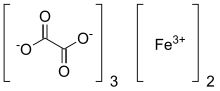
Ferric oxalate
The coordination complex with the formula [Fe(C2O4)3]3- forms a variety of salts, a well-known example being potassium ferrioxalate.
Mössbauer spectrum of Fe2(C2O4)3 · 4 H2O exhibits an isomer shift of 0.38 mm/s and a quadrupole splitting of 0.40 mm/s, suggesting a high spin Fe3+ in octahedral coordination.
Ferric oxalate tetrahydrate has been investigated as a possible cheap material for the positive electrode of lithium-iron batteries.
It can intercalate lithium ions at an average potential of 3.35 V, and has shown a sustainable capacity of 98 mAh/g.
[1] Ferric oxalate hexahydrate is used with sodium borohydride for radical Markovnikov hydrofunctionalization reactions of alkenes.