Nuclear fission product
[1] In fact the short lived products are so predominant that 87 percent decay to stable isotopes within the first month after removal from the reactor core.
This process is the source of so-called delayed neutrons, which play an important role in control of a nuclear reactor.
However, as the fission products approach stable nuclear conditions, the last one or two decays may have a long half-life and release less energy.
A number of these actinides have half lives in the missing range of about 100 to 200,000 years, causing some difficulty with storage plans in this time-range for open cycle non-reprocessed fuels.
Proponents of nuclear fuel cycles which aim to consume all their actinides by fission, such as the Integral Fast Reactor and molten salt reactor, use this fact to claim that within 200 years, their fuel wastes are no more radioactive than the original uranium ore.[3] Fission products emit beta radiation, while actinides primarily emit alpha radiation.
In current nuclear power reactors, about 3% of the uranium in the fuel is converted into fission products as a by-product of energy generation.
Certain fission products decay over seconds to minutes, producing additional delayed neutrons crucial to sustaining criticality.
[9] Operating in this delayed critical state, power changes slowly enough to permit human and automatic control.
This decay heat requires removal after shutdown; loss of this cooling damaged the reactors at Three Mile Island and Fukushima.
However, the very short time scale for the reaction makes a difference in the particular mix of isotopes produced from an atomic bomb.
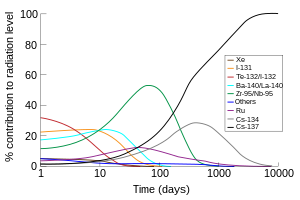
For example, the 134Cs/137Cs ratio provides an easy method of distinguishing between fallout from a bomb and the fission products from a power reactor.
According to Jiri Hala's textbook,[14] the radioactivity in the fission product mixture in an atom bomb is mostly caused by short-lived isotopes such as iodine-131 and barium-140.
In a similar way the release of radio-iodine in a serious power reactor accident could be retarded by adsorption on metal surfaces within the nuclear plant.
Locations where radiation fields once posed immediate mortal threats, such as much of the Chernobyl Nuclear Power Plant on day one of the accident and the ground zero sites of U.S. atomic bombings in Japan (6 hours after detonation) are now relatively safe because the radioactivity has decreased to a low level.
The purpose of radiological emergency preparedness is to protect people from the effects of radiation exposure after a nuclear accident or bomb.
It has been shown that the active iodine released from Chernobyl and Mayak[19] has resulted in an increase in the incidence of thyroid cancer in the former Soviet Union.
One measure which protects against the risk from radio-iodine is taking a dose of potassium iodide (KI) before exposure to radioiodine.
Administering potassium iodide reduces the effects of radio-iodine by 99% and is a prudent, inexpensive supplement to fallout shelters.
Perchlorate ions, a common water contaminant in the USA due to the aerospace industry, has been shown to reduce iodine uptake and thus is classified as a goitrogen.
Perchlorate ions are a competitive inhibitor of the process by which iodide is actively deposited into thyroid follicular cells.
Studies involving healthy adult volunteers determined that at levels above 0.007 milligrams per kilogram per day (mg/(kg·d)), perchlorate begins to temporarily inhibit the thyroid gland's ability to absorb iodine from the bloodstream ("iodide uptake inhibition", thus perchlorate is a known goitrogen).
[20][24] Prophylaxis with perchlorate-containing water at concentrations of 17 ppm, which corresponds to 0.5 mg/kg-day personal intake, if one is 70 kg and consumes 2 litres of water per day, was found to reduce baseline radioiodine uptake by 67%[20] This is equivalent to ingesting a total of just 35 mg of perchlorate ions per day.
In another related study where subjects drank just 1 litre of perchlorate-containing water per day at a concentration of 10 ppm, i.e. daily 10 mg of perchlorate ions were ingested, an average 38% reduction in the uptake of iodine was observed.
Studies of chronically exposed workers though have thus far failed to detect any abnormalities of thyroid function, including the uptake of iodine.
[26] this may well be attributable to sufficient daily exposure or intake of healthy iodine-127 among the workers and the short 8 hr biological half life of perchlorate in the body.
After 80–90 days passed, released radioactive iodine-131 would have decayed to less than 0.1% of its initial quantity, at which time the danger from biouptake of iodine-131 is essentially over.
However, in the event of a radioiodine release too massive and widespread to be controlled by the limited stock of iodide and iodate prophylaxis drugs, then the addition of perchlorate ions to the water supply, or distribution of perchlorate tablets would serve as a cheap, efficacious, second line of defense against carcinogenic radioiodine bioaccumulation.
The cyanide is so tightly bonded to the iron that it is safe for a human to consume several grams of prussian blue per day.
An added advantage of the prussian blue is that the caesium which is stripped from the animal in the droppings is in a form which is not available to plants.
However such treatments with either lime or potash should not be undertaken lightly as they can alter the soil chemistry greatly, so resulting in a change in the plant ecology of the land.