Size-exclusion chromatography
The chromatography column is packed with fine, porous beads which are commonly composed of dextran, agarose, or polyacrylamide polymers.
[5] Even though size exclusion chromatography is widely utilized to study natural organic material, there are limitations.
The advantages of this method include good separation of large molecules from the small molecules with a minimal volume of eluate,[7] and that various solutions can be applied without interfering with the filtration process, all while preserving the biological activity of the particles to separate.
The technique is generally combined with others that further separate molecules by other characteristics, such as acidity, basicity, charge, and affinity for certain compounds.
With size exclusion chromatography, there are short and well-defined separation times and narrow bands, which lead to good sensitivity.
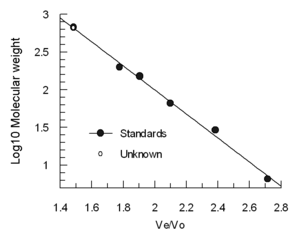
The other advantage to this experimental method is that in certain cases, it is feasible to determine the approximate molecular weight of a compound.
[8][9] Disadvantages are, for example, that only a limited number of bands can be accommodated because the time scale of the chromatogram is short, and, in general, there must be a 10% difference in molecular mass to have a good resolution.
[7] The technique was invented in 1955 by Grant Henry Lathe and Colin R Ruthven, working at Queen Charlotte's Hospital, London.
[14] There were also attempts to fractionate synthetic high polymers; however, it was not until 1964, when J. C. Moore of the Dow Chemical Company published his work on the preparation of gel permeation chromatography (GPC) columns based on cross-linked polystyrene with controlled pore size,[15] that a rapid increase of research activity in this field began.
This process is usually performed within a column, which typically consists of a hollow tube tightly packed with micron-scale polymer beads containing pores of different sizes.
[17] The observed correlation based on the hydrodynamic volume became accepted as the basis of universal SEC calibration.
Still, the use of the hydrodynamic volume, a size based on dynamical properties, in the interpretation of SEC data is not fully understood.
[18] This is because SEC is typically run under low flow rate conditions where hydrodynamic factor should have little effect on the separation.
Based on this theory, it has been shown that the relevant size parameter to the partitioning of polymers in pores is the mean span dimension (mean maximal projection onto a line).
The exclusion limit defines the molecular weight at the upper end of the column 'working' range and is where molecules are too large to get trapped in the stationary phase.
Following are the materials which are commonly used for porous gel beads in size exclusion chromatography [20] And Trade name (kDa) In real-life situations, particles in solution do not have a fixed size, resulting in the probability that a particle that would otherwise be hampered by a pore passing right by it.
Unlike affinity chromatography techniques, a solvent head at the top of the column can drastically diminish resolution as the sample diffuses prior to loading, broadening the downstream elution.
When eluting spectroscopically similar species (such as during biological purification), other techniques may be necessary to identify the contents of each fraction.
It is also possible to analyze the eluent flow continuously with RI, LALLS, Multi-Angle Laser Light Scattering MALS, UV, and/or viscosity measurements.
In general, SEC is considered a low-resolution chromatography as it does not discern similar species very well, and is therefore often reserved for the final step of a purification.
The technique can determine the quaternary structure of purified proteins that have slow exchange times, since it can be carried out under native solution conditions, preserving macromolecular interactions.
Due to the difference in size of two polymers with identical molecular weights, the absolute determination methods are, in general, more desirable.
A typical SEC system can quickly (in about half an hour) give polymer chemists information on the size and polydispersity of the sample.
Likewise, differences between conformation of the analyte and the standard have no effect on an absolute measurement; for example, with MALS analysis, the molar mass of inherently disordered proteins are characterized accurately even though they elute at much earlier times than globular proteins with the same molar mass, and the same is true of branched polymers which elute late compared to linear reference standards with the same molar mass.
In addition, MALS determines the rms radius Rg of molecules above a certain size limit, typically 10 nm.
For smaller molecules, either DLS or, more commonly, a differential viscometer is added to determine hydrodynamic radius and evaluate molecular conformation in the same manner.
Because a correlation function requires anywhere from 3–7 seconds to properly build, a limited number of data points can be collected across the peak.
ASEC with SLS detection is not limited by flow rate and measurement time is essentially instantaneous, and the range of concentration is several orders of magnitude larger than for DLS.
MALS and DLS detectors are often combined in a single instrument for more comprehensive absolute analysis following separation by SEC.