Gene therapy
[4] The first therapeutic use of gene transfer as well as the first direct insertion of human DNA into the nuclear genome was performed by French Anderson in a trial starting in September 1990.
Since that time, further gene therapy drugs were approved, such as alipogene tiparvovec (2012), Strimvelis (2016), tisagenlecleucel (2017), voretigene neparvovec (2017), patisiran (2018), onasemnogene abeparvovec (2019), idecabtagene vicleucel (2021), nadofaragene firadenovec, valoctocogene roxaparvovec and etranacogene dezaparvovec (all 2022).
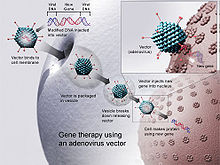
AAVs are characterized by stabilizing the viral capsid, lower immunogenicity, ability to transduce both dividing and nondividing cells, the potential to integrate site specifically and to achieve long-term expression in the in-vivo treatment.
[6] ASO / siRNA approaches such as those conducted by Alnylam and Ionis Pharmaceuticals require non-viral delivery systems, and utilize alternative mechanisms for trafficking to liver cells by way of GalNAc transporters.
[16][33] Following early advances in genetic engineering of bacteria, cells, and small animals, scientists started considering how to apply it to medicine.
[34] Scientists focused on diseases caused by single-gene defects, such as cystic fibrosis, haemophilia, muscular dystrophy, thalassemia, and sickle cell anemia.
Somatic gene therapy represents mainstream basic and clinical research, in which therapeutic DNA (either integrated in the genome or as an external episome or plasmid) is used to treat disease.
[17][66] While the concept of gene replacement therapy is mostly suitable for recessive diseases, novel strategies have been suggested that are capable of also treating conditions with a dominant pattern of inheritance.
[78]: 2647 Animal models suggest that integration of AAV genetic material into the host cell's nuclear genome may cause hepatocellular carcinoma, a form of liver cancer.
Non-viral techniques offer the possibility of repeat dosing and greater tailorability of genetic payloads, which in the future will be more likely to take over viral-based delivery systems.
In academic contexts, a number of laboratories are working on delivery of PEGylated particles, which form serum protein coronas and chiefly exhibit LDL receptor mediated uptake in cells in vivo.
This subject is governed by overlapping regulations from local and federal agencies, including the Department of Health and Human Services, the FDA and NIH's Recombinant DNA Advisory Committee.
This section describes required review processes and other aspects when seeking approval to begin clinical research involving genetic transfer into a human patient.
[142][143][144] For parents, genetic engineering could be seen as another child enhancement technique to add to diet, exercise, education, training, cosmetics, and plastic surgery.
[164] The first approved gene therapy clinical research in the US took place on 14 September 1990, at the National Institutes of Health (NIH), under the direction of William French Anderson.
[167] The treatment of glioblastoma multiforme, the malignant brain tumor whose outcome is always fatal, was done using a vector expressing antisense IGF-I RNA (clinical trial approved by NIH protocol no.1602 24 November 1993,[168] and by the FDA in 1994).
This anti-gene antisense/triple helix therapy has proven to be efficient, due to the mechanism stopping simultaneously IGF-I expression on translation and transcription levels, strengthening anti-tumor immune and apoptotic phenomena.
[176] The mice – which have essentially the same defect that causes human cases – used a viral vector to induce production of fetal hemoglobin (HbF), which normally ceases to be produced shortly after birth.
[194] Cancer immunogene therapy using modified antigene, antisense/triple helix approach was introduced in South America in 2010/11 in La Sabana University, Bogota (Ethical Committee 14 December 2010, no P-004-10).
[201][202] In 2011, Neovasculgen was registered in Russia as the first-in-class gene-therapy drug for treatment of peripheral artery disease, including critical limb ischemia; it delivers the gene encoding for VEGF.
[217] In July researchers reported promising results for six children with two severe hereditary diseases had been treated with a partially deactivated lentivirus to replace a faulty gene and after 7–32 months.
[220][221] In October researchers reported that two children born with adenosine deaminase severe combined immunodeficiency disease (ADA-SCID) had been treated with genetically engineered stem cells 18 months previously and that their immune systems were showing signs of full recovery.
One of the four trials did find weak evidence that liposome-based CFTR gene transfer therapy may lead to a small respiratory improvement for people with CF.
[244] In February Kite Pharma announced results from a clinical trial of CAR-T cells in around a hundred people with advanced non-Hodgkin lymphoma.
[249][250] On 13 November, medical scientists working with Sangamo Therapeutics, headquartered in Richmond, California, announced the first ever in-body human gene editing therapy.
[268] In May, onasemnogene abeparvovec (Zolgensma) was approved by the European Union for the treatment of spinal muscular atrophy in people who either have clinical symptoms of SMA type 1 or who have no more than three copies of the SMN2 gene, irrespective of body weight or age.
[269] In August, Audentes Therapeutics reported that three out of 17 children with X-linked myotubular myopathy participating the clinical trial of a AAV8-based gene therapy treatment AT132 have died.
[300] In June 2023, the FDA gave an accelerated approval to Elevidys for Duchenne muscular dystrophy (DMD) only for boys 4 to 5 years old as they are more likely to benefit from the therapy which consists of one-time intravenous infusion of a virus (AAV rh74 vector) that delivers a functioning "microdystrophin" gene (138 kDa) into the muscle cells to act in place of the normal dystrophin (427 kDa) that is found mutated in this disease.
[108] 2024 In November 2024, FDA granted accelerated approval for eladocagene exuparvovec-tneq (Kebilidi, PTC Therapeutics), a direct-to-brain gene therapy for aromatic L-amino acid decarboxylase deficiency.
[302] It uses a recombinant adeno-associated virus serotype 2 (rAAV2) to deliver a functioning DOPA decarboxylase (DDC) gene directly into the putamen, increasing the AADC enzyme and restoring dopamine production.