Hartree–Fock method
By invoking the variational method, one can derive a set of N-coupled equations for the N spin orbitals.
In deriving what is now called the Hartree equation as an approximate solution of the Schrödinger equation, Hartree required the final field as computed from the charge distribution to be "self-consistent" with the assumed initial field.
The Hartree–Fock method finds its typical application in the solution of the Schrödinger equation for atoms, molecules, nanostructures[3] and solids but it has also found widespread use in nuclear physics.
In atomic structure theory, calculations may be for a spectrum with many excited energy levels, and consequently, the Hartree–Fock method for atoms assumes the wave function is a single configuration state function with well-defined quantum numbers and that the energy level is not necessarily the ground state.
For both atoms and molecules, the Hartree–Fock solution is the central starting point for most methods that describe the many-electron system more accurately.
The rest of this article will focus on applications in electronic structure theory suitable for molecules with the atom as a special case.
The origin of the Hartree–Fock method dates back to the end of the 1920s, soon after the discovery of the Schrödinger equation in 1926.
By introducing the quantum defect d as an empirical parameter, the energy levels of a generic atom were well approximated by the formula
These early researchers later introduced other potentials containing additional empirical parameters with the hope of better reproducing the experimental data.
In 1927, D. R. Hartree introduced a procedure, which he called the self-consistent field method, to calculate approximate wave functions and energies for atoms and ions.
[4] Hartree sought to do away with empirical parameters and solve the many-body time-independent Schrödinger equation from fundamental physical principles, i.e., ab initio.
[5][6] In 1930, Slater and V. A. Fock independently pointed out that the Hartree method did not respect the principle of antisymmetry of the wave function.
[7] [8] The Hartree method used the Pauli exclusion principle in its older formulation, forbidding the presence of two electrons in the same quantum state.
A solution to the lack of anti-symmetry in the Hartree method came when it was shown that a Slater determinant, a determinant of one-particle orbitals first used by Heisenberg and Dirac in 1926, trivially satisfies the antisymmetric property of the exact solution and hence is a suitable ansatz for applying the variational principle.
Fock's original method relied heavily on group theory and was too abstract for contemporary physicists to understand and implement.
Even so, calculating a solution by hand using the Hartree–Fock equations for a medium-sized atom was laborious; small molecules required computational resources far beyond what was available before 1950.
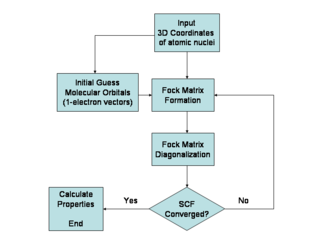
The Hartree–Fock method is typically used to solve the time-independent Schrödinger equation for a multi-electron atom or molecule as described in the Born–Oppenheimer approximation.
The starting point for the Hartree–Fock method is a set of approximate one-electron wave functions known as spin-orbitals.
The orbitals are optimized by requiring them to minimize the energy of the respective Slater determinant.
In this way, the Hartree–Fock orbitals are optimized iteratively until the change in total electronic energy falls below a predefined threshold.
Following the basic postulates of quantum mechanics, the Hartree–Fock wave function can then be used to compute any desired chemical or physical property within the framework of the Hartree–Fock method and the approximations employed.
Performing the variation, we obtain The factor 1/2 before the double integrals in the molecular Hamiltonian drops out due to symmetry and the product rule.
Although Hartree-Fock equation appears in the form of a eigenvalue problem, the Fock operator itself depends on
However, in most modern computer programs for molecular Hartree–Fock calculations this procedure is not followed due to the high numerical cost of orthogonalization and the advent of more efficient, often sparse, algorithms for solving the generalized eigenvalue problem, of which the Roothaan–Hall equations are an example.
A clever dodge, employed by Hartree, for atomic calculations was to increase the nuclear charge, thus pulling all the electrons closer together.
Modern molecular Hartree–Fock computer programs use a variety of methods to ensure convergence of the Roothaan–Hall equations.
Neglect of electron correlation can lead to large deviations from experimental results.
A number of approaches to this weakness, collectively called post-Hartree–Fock methods, have been devised to include electron correlation to the multi-electron wave function.
Others expand the true multi-electron wave function in terms of a linear combination of Slater determinants—such as multi-configurational self-consistent field, configuration interaction, quadratic configuration interaction, and complete active space SCF (CASSCF).
An alternative to Hartree–Fock calculations used in some cases is density functional theory, which treats both exchange and correlation energies, albeit approximately.