Hepatitis B virus
[3] The genus is classified as part of the Hepadnaviridae family, which contains four other genera, Avihepadnavirus, Herpetohepadnavirus, Metahepadnavirus and Parahepadnavirus.
The virus is divided into four major serotypes (adr, adw, ayr, ayw) based on antigenic epitopes present on its envelope proteins.
The viral strains have also been divided into ten genotypes (A–J) and forty subgenotypes according to overall nucleotide sequence variation of the genome.
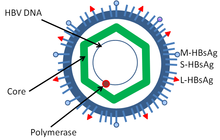
[13] The outer envelope contains embedded proteins which are involved in viral binding of, and entry into, susceptible cells.
[28] Avihepadnaviruses lack the X protein but a vestigial X reading frame is present in the genome of duck hepadnavirus.
[30][31][32] In 2021, a study reconstructed 137 ancient HBV genomes and proved the presence of the virus in humans since at least 10,000 years.
[30][31][32] HBV strains found in African and South-East Asian apes (chimpanzees, gorillas, orangutans, bonobos and gibbons) appear related to human HBV strains, which could reflect past cross-species transmission events.
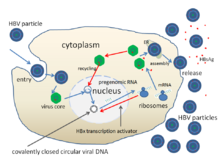
The viral genes are transcribed by the cellular RNA polymerase II in the cell nucleus from a covalently closed circular DNA (cccDNA) template.
Both enhancers exhibit greater activity in cells of hepatic origin, and together they drive and regulate the expression of the complete viral transcripts.
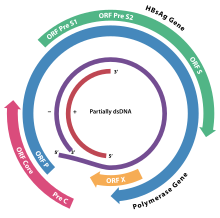
The HBsAg gene is one long open reading frame but contains three in frame "start" (ATG) codons that divide the gene into three sections, pre-S1, pre-S2, and S. Because of the multiple start codons, polypeptides of three different sizes called large (pre-S1 + pre-S2 + S), middle (pre-S2 + S), and small (S) are produced.
Interestingly, a 40 kDa X-Core fusion protein is encoded by a long viral 3.9-kb transcript, whose function remains unclear.
[4] Viral infection by hepatitis B virus (HBV) causes many hepatocyte changes due to the direct action of a protein encoded by the virus, HBx, and to indirect changes due to a large increase in intracellular reactive oxygen species (ROS) after infection.
HBx causes dysregulation in part by binding to genomic DNA, changing expression patterns of miRNAs, affecting histone methyltransferases, binding to SIRT1 protein to activate transcription, and cooperating with histone methylases and demethylases to change cell expression patterns.
[57] HBx is partly responsible for the approximate 10,000-fold increase in intracellular reactive oxygen species (ROS) upon chronic HBV infection.
[61] Epigenetic alterations and mutations may cause defects in the cellular machinery that then contribute to liver disease.
[57][65] In addition to protein coding genes, about 15 microRNAs and 16 Long non-coding RNAs are also affected by the binding of HBx to their promoters.
The origin of hepatitis B virus can be traced back to the 5th century BCE and is even mentioned in Babylonian clay tablets.
[66][67] However, due to the lengthy time interval, measured in weeks, between exposure of the causative agent and the development of illness prevented recognition of jaundice as an infectious disease until the 20th century.
[68] The first recorded cases of hepatitis B virus infection occurred in 1883 after the smallpox vaccine containing human lymph was administered to a group of people.
This was one of the first breakthroughs in the effort to understand the pathology of viral hepatitis that instigated jaundice in those infected with HBV.
Hepatitis B virus is more prominently found in US citizens of Asian, Pacific Islander, or African descent and roughly 25% of these individuals will receive a diagnosis.
Every year, an estimated 820,000 people die from hepatitis B infection and related HBV complications.
[76] The spread of HBV during pregnancy remains the highest risk for developing chronic hepatitis B later in childhood.
[78] The spread of hepatitis B virus in the western world occurs most often through sexual intercourse or needle sharing by intravenous drug users (IVDU).
(need reference) The spread of hepatitis B virus occurs most often through vertical transmission from mother to child during birth and delivery.
HBV can also be spread through contact with blood or other bodily fluids during sexual intercourse with an infected partner.
Although HBV can be infectious on surfaces for up to seven days, it is not spread through breastfeeding, sharing eating utensils, hugging, kissing, holding hands, coughing, or sneezing.
Most cases of HBV and HCV co-infection occurs among Intravenous drug users, unscreened blood products, or exposure to dirty needles and unsterilized medical equipment.
Reporting of this co-infection may be underreported due to hepatitis C's ability to become the dominant liver virus during coinfection, reducing the detectable amount of HBV found in the body.
Hepatitis B infection is one of the leading causes of hospitalizations and death among patients with HIV since the development and use of antiretroviral therapies.