Hepatitis C virus
[13] Although HVR1 is quite variable in amino acid sequence, this region has similar chemical, physical, and conformational characteristics across many E2 glycoproteins.
The core domain of the HCV IRES contains a four-way helical Holliday junction that is integrated within a predicted pseudoknot.
[17] The conformation of this core domain constrains the open reading frame's orientation for positioning on the 40S ribosomal subunit.
The remaining cleavages downstream from this site are catalysed by a serine protease also contained within the N-terminal region of NS3.
The virus may also replicate in peripheral blood mononuclear cells, potentially accounting for the high levels of immunological disorders found in chronically infected HCV patients.
[10] HCV has a wide variety of genotypes and mutates rapidly due to a high error rate on the part of the virus' RNA-dependent RNA polymerase.
[29] Entry into host cells occur through complex interactions between virions, especially through their glycoproteins, and cell-surface molecules CD81, LDL receptor, SR-BI, DC-SIGN, Claudin-1, and Occludin.
Claudin 1, which is a tight-junction protein, and CD81 link to create a complex, priming them for later HCV infection processes.
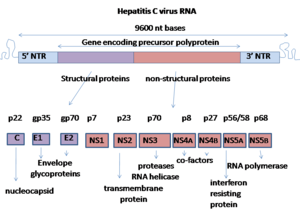
The polyprotein is then proteolytically processed by viral and cellular proteases to produce three structural (virion-associated) and seven nonstructural (NS) proteins.
The NS proteins then recruit the viral genome into an RNA replication complex, which is associated with rearranged cytoplasmic membranes.
[35] The core protein associates with lipid droplets and utilises microtubules and dyneins to alter their location to a perinuclear distribution.
[37] Another hypothesis states that the viral particle may be secreted from the endoplasmic reticulum through the endosomal sorting complex required for transport (ESCRT) pathway.
[44] HCV genotypes 1 and 4 have been distributed endemically in overlapping areas of West and Central Africa, infecting for centuries human populations carrying the genetic polymorphism in question.
[48] Because of this mode of spread the key groups at risk are intravenous drug users (IDUs), recipients of blood products and sometimes patients on haemodialysis.
[48] It has also been argued that given the extremely prolonged periods of persistence of HCV in humans, even very low and undetectable rates of mechanical transmission via biting insects may be sufficient to maintain endemic infection in the tropics, where people receive large number of insect bites.
[51] A Bayesian analysis suggests that the major genotypes diverged about 300–400 years ago from the common ancestor virus.
[citation needed] A study of genotype 6 strains suggests an earlier date of evolution: approximately 1,100 to 1,350 years Before Present.
[53] A study of European, US and Japanese isolates suggested that the date of origin of genotype 1b was approximately in the year 1925.
The genotype 2 strains from Africa can be divided into four clades that correlate with their country of origin: (1) Cameroon and Central African Republic (2) Benin, Ghana and Burkina Faso (3) Gambia, Guinea, Guinea-Bissau and Senegal (4) Madagascar.
[57] There is also strong evidence for the dissemination of HCV genotype 2 from West Africa to the Caribbean by the trans-Atlantic slave trade.
[59] These dates from these various countries suggests that this virus may have evolved in South East Asia and was spread to West Africa by traders from Western Europe.
Once introduced to a country its spread has been influenced by many local factors including blood transfusions, vaccination programmes, intravenous drug use and treatment regimes.
Additional work is required to determine the dates of evolution of the various genotypes and the timing of their spread across the globe.
[63] HCV, as with most RNA viruses, exists as a viral quasispecies, making it very difficult to isolate a single strain or receptor type for study.
[64][65] Current research is focused on small-molecule inhibitors of the viral protease, RNA polymerase and other nonstructural genes.
It has been reported to be the first drug that has demonstrated safety and efficacy to treat certain types of HCV infection without the need for co-administration of interferon.
Oxymatrine, for example, is a root extract found in the continent of Asia that has been reported to have antiviral activity against HCV in cell cultures and animal studies.
Alter, Michael Houghton, and Charles M. Rice had been awarded the 2020 Nobel Prize in Physiology or Medicine for the discovery of HCV.