Hydrogen isotope biogeochemistry
Understanding isotopic fingerprints and the sources of fractionation that lead to variation between them can be applied to address a diverse array of questions ranging from ecology and hydrology to geochemistry and paleoclimate reconstructions.
Urey and his colleague George Murphy calculated the redshift of heavy hydrogen from the Balmer series and observed very faint lines on a spectrographic study.
To intensify the spectroscopic lines for publishable data, Murphy and Urey paired with Ferdinand Brickwedde and distilled a more concentrated pool of heavy hydrogen, now called deuterium.
At the end of World War II, physical chemist Willard Libby detected the residual radioactivity of a tritium sample with a Geiger counter,[4] providing a more accurate understanding of the half-life, now accepted as 12.3 years.
A landmark paper in 1980 by Marilyn Epstep, now M. Fogel, and Thomas Hoering titled "Biogeochemistry of the stable hydrogen isotopes" refined the links between organic materials and sources.
Hydrogen isotope applications quickly emerged in petroleum geochemistry by measuring oil, paleoclimatology by observing lipid biomarkers, and ecology by constructing trophic dynamics.
Large D/H differences, of thousands of ‰, can be found between Earth and other planetary bodies such as Mars, likely due to variations in isotope fractionation during planet formation and the loss of hydrogen into space.
[46] For some time, researchers believed that large hydrocarbon molecules were impervious to hydrogen exchange, but recent work has identified many reactions that allow isotope reordering.
[60] A vast portion of global atmospheric water vapor comes from the Western Pacific near the tropics, (mean 2009) and the HIC of air depends on temperature and humidity.
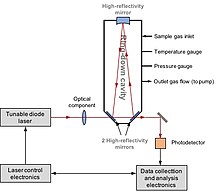
This is for several reasons with regard to hydrogen: The classical offline preparation for the conversion is combustion over CuO at >800°C in sealed quartz tubes, followed by the isolation of resulting water and the reduction to H2 over hot metal at 400 ~1000°C on a vacuum line.
In 1999, Hilkert et al. developed a robust method by integrating the high temperature conversion (TC) into GC-IRMS and adding a pre-cup electrostatic sector and a retardation lens in front of the m/z=3 cup collector.
In contrast to temperature control at high latitudes, the isotopic composition of precipitation in the tropics is mainly influenced by rainfall amount (negative correlation).
[150] Reconstructions of rainfall amount in the tropics in the geological past are mostly based on δ18O of speleothems[151][152] or δD of biogenic lipids,[153][154] both of which are thought of as proxies for the isotopic composition of precipitation.
[159] The δD-temperature (of the inversion layer where snow forms) conversion in east Antarctica based on modern spatial gradient of δD (9‰/°C) is ΔTI=(ΔδDice-8Δδ18Osw)/9, which takes into account variations in seawater isotopic composition caused by global ice volume changes.
[167] Nevertheless, the Vostok ice core record shows some very important results: (1) A consistent δD depletion of ~70‰ during the last four glacial periods compared to interglacial times, corresponding to a cooling of 8°C in Antarctica; (2) A consistent drop of atmospheric CO2 concentration by 100 ppmv and CH4 drop by ~300 ppbv during glacial times relative to interglacials, suggesting a role of greenhouse gases in regulating global climate; (3) Antarctic air temperature and greenhouse gas concentration changes precede global ice volume and Greenland air temperature changes during glacial terminations, and greenhouse gases may be an amplifier of insolation forcing during glacial-interglacial cycles.
[173] Finally, preservation of biomolecules in the geologic record does not faithfully represent whole ecosystems, and there is always the threat of hydrogen exchange, particularly if the sediments are subjected to high temperatures.
[173] With ever-improving measurement techniques for single molecules, and correlation with other independent proxies in the geological record that can help constrain some variables, investigating the HIC of leaf waxes can be extremely productive.
Leaf wax δD data has been successfully applied to improving our understanding of climate driven changes in terrestrial hydrology, by showing that ocean circulation and surface temperature have a significant effect on continental precipitation.
Both the δDs of sea water and the fractionations associated with hyptophyte biochemistry (εbio) are fairly well understood, so alkenones can be readily used to observe the secondary effect of salinity on δD.
[209] Lab experiments with clay minerals have shown that the hydrogen and oxygen isotope compositions are relatively resistant to alteration at moderate temperature (<100°C), and can preserve the original meteoric water signal.
[209] In one such study, an isotope enrichment was observed in smectite on the east side of the Sierra Nevada in California from mid-Miocene to late Pliocene, suggesting a decrease in elevation during this period.
The HIC signal of fossil fuels results from both inheritance of source material and water as well as fractionations during hydrocarbon generation and subsequent alteration by processes such as isotopic exchange or biodegradation.
The study of hydrogen isotopes of fossil fuels has been applied as proxies and tools in the following aspects: The first stage that sedimentary organic matter (SOM) experiences after deposition is diagenesis.
Jon Telling et al., synthesized isotopically reversed (in both C and H) low-molecular alkanes using gas-phase radical recombination reactions in electrical discharge experiments, providing another possible mechanism.
Analyzing HIC at the compound level avoids problems from differences in exchange rates, simplifies sources and products relationships, and draws a much more detailed picture.
Further experiments on unicellular algae Eudorina unicocca and Volvox aureus show no effect of growth rate (controlled by nitrogen limitation) on fatty acid δD.
Also, analysis of o-xylene in a polluted site showed high residual DHRs after biodegradation, consistent with activation of C-H bonds being a rate limiting step in this process[279] Stable isotope ratios have found uses in various instances where the authenticity or origin of a chemical compound is called into question.
This concern has been addressed in a recent study which concluded that the effect of DHR of drinking water did not pose an insurmountable source of error for this anti-doping testing strategy.
[288] DHRs can also be determined for specific sites in a drug by 2H NMR, and has been used to distinguish between different synthetic methods for ibuprofen and naproxen in one study,[289] and prozac and fluoxetine in another.
[290] These studies show that bulk DHR information for EA-IRMS, and site-specific DHRs from 2H NMR have great utility for pharmaceutical drug authenticity testing.