Hydrogen production
The global hydrogen generation market was fairly valued at US$155 billion in 2022, and expected to grow at a compound annual growth rate of 9.3% from 2023 to 2030.
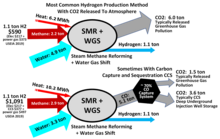
The former carrier consumes the fossil resource and in the steam methane reforming (SMR) process produces greenhouse gas carbon dioxide.
Due to their use of water, a readily available resource, electrolysis and similar water-splitting methods have attracted the interest of the scientific community.
[54] Traditionally, alkaline electrolysers are cheaper in terms of investment (they generally use nickel catalysts), but less-efficient; PEM electrolysers, conversely, are more expensive (they generally use expensive platinum group metal catalysts) but are more efficient and can operate at higher current densities, and can therefore be possibly cheaper if the hydrogen production is large enough.
[56] These cells have the advantage of being comparatively simple and can be designed to accept widely varying voltage inputs, which makes them ideal for use with renewable sources of energy such as photovoltaic solar panels.
The lower the energy used by a generator, the higher would be its efficiency; a 100%-efficient electrolyser would consume 39.4 kilowatt-hours per kilogram (142 MJ/kg) of hydrogen,[61] 12,749 joules per litre (12.75 MJ/m3).
Practical electrolysis typically uses a rotating electrolyser, where centrifugal force helps separate gas bubbles from water.
The US DOE target price for hydrogen in 2020 is $2.30/kg, requiring an electricity cost of $0.037/kWh, which is achievable given recent PPA tenders for wind and solar in many regions.
The chemical reaction takes the general form: Idealized examples for heating oil and coal, assuming compositions C12H24 and C24H12 respectively, are as follows: The Kværner process or Kvaerner carbon black and hydrogen process (CB&H)[88] is a plasma pyrolysis method, developed in the 1980s by a Norwegian company of the same name, for the production of hydrogen and carbon black from liquid hydrocarbons (CnHm).
[95][96] In the Mponeng gold mine, South Africa, researchers found bacteria in a naturally occurring high radiation zone.
[97] Water spontaneously dissociates at around 2500 °C, but this thermolysis occurs at temperatures too high for usual process piping and equipment resulting in a rather low commercialization potential.
The high-temperature gas-cooled reactor (HTGR) is one of the most promising CO2-free nuclear technique to produce hydrogen by splitting water in a large scale.
In this method, iodine-sulfur (IS) thermo-chemical cycle for splitting water and high-temperature steam electrolysis (HTSE) were selected as the main processes for nuclear hydrogen production.
Thermochemical cycles combine solely heat sources (thermo) with chemical reactions to split water into its hydrogen and oxygen components.
However, if this process is assisted by photocatalysts suspended directly in water instead of using photovoltaic and an electrolytic system the reaction is in just one step, it can be made more efficient.
In addition, a wide variety of waste and low-value materials such as agricultural biomass as renewable sources can be utilized to produce hydrogen via biochemical or thermochemical pathways.
For example, studies on hydrogen production using H. salinarium, an anaerobic photosynthetic bacteria, coupled to a hydrogenase donor like E. coli, are reported in literature.
Dark fermentation reactions do not require light energy, so they are capable of constantly producing hydrogen from organic compounds throughout the day and night.
With biocatalysed electrolysis, hydrogen is generated after running through the microbial fuel cell and a variety of aquatic plants Archived 2010-05-17 at the Wayback Machine can be used.
[148] European largest (1 400 000 kg/a, High-pressure Electrolysis of water, alkaline technology) hydrogen production plant is operating at Kokkola, Finland.
Water is broken into hydrogen and oxygen by electrolysis – a photoelectrochemical cell (PEC) process which is also named artificial photosynthesis.
[152] William Ayers at Energy Conversion Devices demonstrated and patented the first multijunction high efficiency photoelectrochemical system for direct splitting of water in 1983.
If this process is assisted by photocatalysts suspended directly in water instead of using photovoltaic and an electrolytic system, the reaction is in just one step, which can improve efficiency.
[154][155] A method studied by Thomas Nann and his team at the University of East Anglia consists of a gold electrode covered in layers of indium phosphide (InP) nanoparticles.
They introduced an iron-sulfur complex into the layered arrangement, which when submerged in water and irradiated with light under a small electric current, produced hydrogen with an efficiency of 60%.
[156] In 2015, it was reported that Panasonic Corp. has developed a photocatalyst based on niobium nitride that can absorb 57% of sunlight to support the decomposition of water to produce hydrogen gas.
Hydrosol-2 is a 100-kilowatt pilot plant at the Plataforma Solar de Almería in Spain which uses sunlight to obtain the required 800 to 1,200 °C to heat water.
[169][170] Blue hydrogen has been estimated to have a greenhouse gas footprint that is 20% greater than burning gas or coal for heat and 60% greater when compared to burning diesel for heat, assuming US up- and mid-stream methane leakage rates and production via steam methane reformers (SMR) retrofitted with carbon dioxide capture.
The Oskarshamn Nuclear Power Plant made an agreement in January 2022 to supply commercial pink hydrogen in the order of kilograms per day.
Although requiring expensive technologies, hydrogen can be cooled, compressed and purified for use in other processes on site or sold to a customer via pipeline, cylinders or trucks.