Hydronium
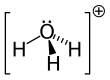
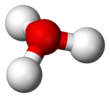
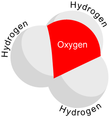
In chemistry, hydronium (hydroxonium in traditional British English) is the cation [H3O]+, also written as H3O+, the type of oxonium ion produced by protonation of water.
In fact, acids must be surrounded by more than a single water molecule in order to ionize, yielding aqueous H+ and conjugate base.
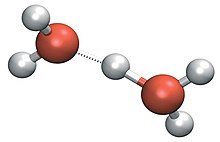
Because the base of the pyramid is made up of three identical hydrogen atoms, the H3O+ molecule's symmetric top configuration is such that it belongs to the C3v point group.
[13] On the other hand, Silverstein has shown that Ballinger and Long's experimental results [14] support a pKa of 0.0 for the aqueous proton.
[17] Virtually all such free protons are quickly hydrated; acidity of an aqueous solution is therefore more accurately characterized by its concentration of H+(aq).
[19] However, more recent ab initio method molecular dynamics simulations have shown that, on average, the hydrated proton resides on the surface of the H3O+(H2O)20 cluster.
[21] In the Zundel H5O+2 complex the proton is shared equally by two water molecules in a symmetric hydrogen bond.
[22] A work in 1999 indicates that both of these complexes represent ideal structures in a more general hydrogen bond network defect.
[23] Isolation of the hydronium ion monomer in liquid phase was achieved in a nonaqueous, low nucleophilicity superacid solution (HF−SbF5SO2).
For example, nitric acid has an ionization constant of 101.4, and mixtures with water at all proportions are liquid at room temperature.
[27] X-ray crystallography shows a C3v symmetry for the hydronium ion with each proton interacting with a bromine atom each from three carborane anions 320 pm apart on average.
In crystals grown from a benzene solution the solvent co-crystallizes and a H3O·(C6H6)3 cation is completely separated from the anion.
In the cation three benzene molecules surround hydronium forming pi-cation interactions with the hydrogen atoms.
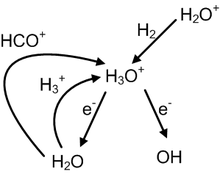
[33] H3O+ can produce either OH− or H2O through dissociative recombination reactions, which occur very quickly even at the low (≥10 K) temperatures of dense clouds.
Astronomers are especially interested in determining the abundance of water in various interstellar climates due to its key role in the cooling of dense molecular gases through radiative processes.
[36] Hydronium, on the other hand, has several transitions that make it a superior candidate for detection and identification in a variety of situations.
[36] This information has been used in conjunction with laboratory measurements of the branching ratios of the various H3O+ dissociative recombination reactions[34] to provide what are believed to be relatively accurate OH− and H2O abundances without requiring direct observation of these species.
Note that the rates of these six reactions are such that they make up approximately 99% of hydronium ion's chemical interactions under these conditions.
[42] However, before an astronomical search could be underway there was still the matter of determining hydronium's spectroscopic features in the gas phase, which at this point were unknown.