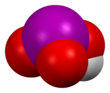
Iodic acid
The structure consists of pyramidal molecules linked by hydrogen bonding and intermolecular iodine-oxygen interactions.
When iodic acid acts as oxidizer, then the product of the reaction is either iodine, or iodide ion.
Under some special conditions (very low pH and high concentration of chloride ions, such as in concentrated hydrochloric acid), iodic acid is reduced to iodine trichloride, a golden yellow compound in solution and no further reduction occurs.
Iodic acid can be used to synthesize sodium or potassium iodate for increasing iodine content of salt.
[citation needed] Iodic acid is part of a series of oxyacids in which iodine can assume oxidation states of −1, +1, +3, +5, or +7.