Cementite
[4] It is a hard, brittle material,[4] normally classified as a ceramic in its pure form, and is a frequently found and important constituent in ferrous metallurgy.
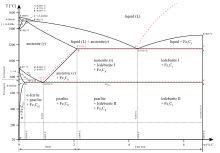
The name cementite originated from the theory of Floris Osmond and J. Werth, in which the structure of solidified steel consists of a kind of cellular tissue, with ferrite as the nucleus and Fe3C the envelope of the cells.
This morphological difference influences the rate of austenite formation and decomposition, with fine cementite promoting faster transformations due to its increased surface area and the proximity of the carbide-ferrite interface.
Furthermore, the dissolution kinetics of cementite during annealing are slower for coarse carbides, impacting the microstructural evolution during heat treatments.
These include epsilon (ε) carbide, hexagonal close-packed Fe2–3C, precipitates in plain-carbon steels of carbon content > 0.2%, tempered at 100–200 °C.