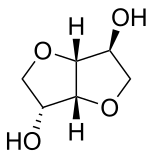
Isosorbide
Isosorbide is a bicyclic chemical compound from the group of diols and the oxygen-containing heterocycles, containing two fused furan rings.
The starting material for isosorbide is D-sorbitol, which is obtained by catalytic hydrogenation of D-glucose, which is in turn produced by hydrolysis of starch.
[1][2] Because of its pronounced hygroscopicity, isosorbide is used as a humectant and in medicine as an osmotic diuretic for the treatment of hydrocephalus and acute angle-closure glaucoma.
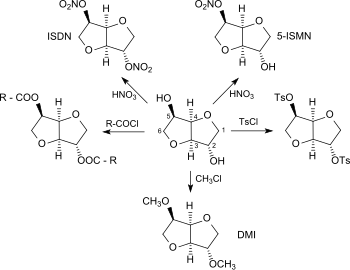
As a diol, isosorbide can be mono- or biderivatized using the standard methods of organic chemistry, such as nitration, esterification, etherification, tosylation, etc., and converted into compounds with interesting properties or into monomeric units for novel polymers.
2,5-isosorbide dinitrate is suitable (just like its major metabolite 5-isosorbide mononitrate, ISMN[6]) for the treatment of angina pectoris due to its vasodilator effect.
So far, 1,2,5,6,9,10-hexabromocyclododecane (HBCD) has been widely used as a flame retardant in extruded polystyrene foam (XPS) in the construction and insulation sector, but it was as SVHC (substance of very high concern) banned from manufacturing and application in May 2013.
Therefore, today's polyesters with isosorbide and monoethylene glycol are examined as diol components that show improved properties such as less discoloration.
Isosorbide bis-glycidyl ether can be crosslinked to a thermosetting epoxy resins with suitable curing agents, such as polyamines or cyclic acid anhydrides.
The hydroxy group in 5-position is endo oriented and forms a hydrogen bond with the oxygen atom in the adjacent furan ring.