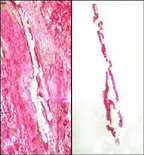
Laser capture microdissection
Laser-capture microdissection (LCM) is a method to procure subpopulations of tissue cells under direct microscopic visualization.
The total time required to carry out this protocol is typically 1–1.5 h.[3] A laser is coupled into a microscope and focuses onto the tissue on the slide.
The heat is introduced, for example, by a red or infrared (IR) laser onto a membrane stained with an absorbing dye.
[4] Under a microscope using a software interface, a tissue section (typically 5-50 micrometres thick) is viewed and individual cells or clusters of cells are identified either manually or in semi-automated or more fully automated ways allowing the imaging and then automatic selection of targets for isolation.
[6] Another process follows gravity-assisted microdissection method that turns on gravity to collect samples in tube cap under the slide used (used by ION LMD system, Jungwoo F&B).
In case of this system, it moves the motorized stage to cut the cells of interests, keeping the laser beam fixed.
And the system uses a 355 nm Solid-state laser(UV-A) which is the safest way to cut the tissues without RNA or DNA damage.
The area to be isolated when a near-IR laser to activate transfer film on a cap placed on the tissue sample, melting the adhesive which then fuses the film with the underlying cells of choice (see Arcturus systems); and/or by activating a UV laser to cut out the cell of interest.
[9] The fourth UV based technology (used by Molecular Machines and Industries AG) offers a slight difference to the 3rd technology here by essentially creating a sandwich of sorts with slide>sample>and membrane overlying the sample by the use of a frame slide whose membrane surface is cut by the laser and ultimately picked up from above by a special adhesive cap.
The laser capture microdissection process does not alter or damage the morphology and chemistry of the sample collected, nor the surrounding cells.
For this reason, LCM is a useful method of collecting selected cells for DNA, RNA and/or protein analyses.