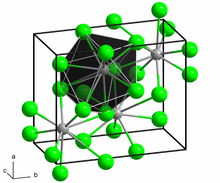
Lead(II) chloride
In the gas phase, PbCl2 molecules have a bent structure with the Cl–Pb–Cl angle being 98° and each Pb–-Cl bond distance being 2.44 Å.
[7] Such PbCl2 is emitted from internal combustion engines that use ethylene chloride-tetraethyllead additives for antiknock purposes.
PbCl2 reacts with molten NaNO2 to give PbO: PbCl2 is used in synthesis of lead(IV) chloride (PbCl4): Cl2 is bubbled through a saturated solution of PbCl2 in aqueous NH4Cl forming [NH4]2[PbCl6].
[11] The usual alkylating agents are employed, including Grignard reagents and organolithium compounds: These reactions produce derivatives that are more similar to organosilicon compounds, i.e. that Pb(II) tends to disproportionate upon alkylation.
PbCl2 can be used to produce PbO2 by treating it with sodium hypochlorite (NaClO), forming a reddish-brown precipitate of PbO2.