Lithium bis(trimethylsilyl)amide
Lithium bis(trimethylsilyl)amide is a lithiated organosilicon compound with the formula LiN(Si(CH3)3)2.
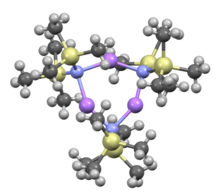
Like many lithium reagents, it has a tendency to aggregate and will form a cyclic trimer in the absence of coordinating species.
S(N(Si(CH3)3)2)2 is prepared by the reaction of lithium bis(trimethylsilyl)amide and sulfur dichloride (SCl2).
The S(N(Si(CH3)3)2)2 reacts with the combination of SCl2 and sulfuryl chloride (SO2Cl2) to form S4N4, trimethylsilyl chloride, and sulfur dioxide:[5] Li(HMDS) can react with a wide range of metal halides, by a salt metathesis reaction, to give metal bis(trimethylsilyl)amides.
With ammonia as donor base lithium bis(trimethylsilyl)amide forms a trisolvated monomer that is stabilized by intermolecular hydrogen bonds.