Lithium borate
A colorless solid, lithium borate is used in making glasses and ceramics.
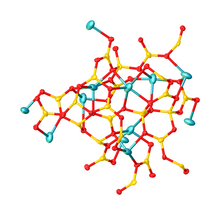
Its structure consists of a polymeric borate backbone.
The Li+ centers are bound to four and five oxygen ligands.
[1][2] Lithium borate can be used in the laboratory as LB buffer for gel electrophoresis of DNA and RNA.
It is also used in the borax fusion method to vitrify mineral powder specimens for analysis by WDXRF spectroscopy.