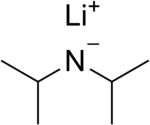
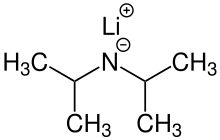
Lithium diisopropylamide
Lithium diisopropylamide (commonly abbreviated LDA) is a chemical compound with the molecular formula LiN(CH(CH3)2)2.
It is used as a strong base and has been widely utilized due to its good solubility in non-polar organic solvents and non-nucleophilic nature.
It was first prepared by Hamell and Levine in 1950 along with several other hindered lithium diorganylamides to effect the deprotonation of esters at the α position without attack of the carbonyl group.
As such it is commercially available as a solution in polar aprotic solvents such as THF and ether; however, for small scale use (less than 50 mmol), it is common and more cost effective to prepare LDA in situ.
A weaker base such as an alkoxide, which reversibly deprotonates the substrate, affords the more thermodynamically stable benzylic enolate.