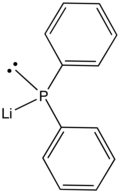
Lithium diphenylphosphide
It is a red, air-sensitive solid that is used in the preparation of diphenylphosphino compounds.
The lithium, sodium, and potassium salts are prepared by reduction of chlorodiphenylphosphine,[1] triphenylphosphine,[2][3] or tetraphenyldiphosphine with alkali metals (M): They can also be obtained by deprotonation of diphenylphosphine.
With water, the salts convert to diphenylphosphine:[3] With halocarbons, the salts react to give tertiary phosphines:[4] When treated with metal halides, lithium diphenylphosphide gives transition metal phosphido complexes.
Although treated as salts, alkali diphenylphosphides are highly aggregated in solution.
As an ether complex, the lithium salt is dark red.