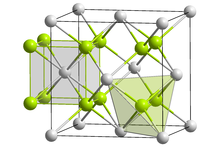
Lithium sulfide
Lithium sulfide is the inorganic compound with the formula Li2S.
It forms a solid yellow-white deliquescent powder.
In air, it easily hydrolyses to release foul smelling hydrogen sulfide gas.
This reaction is conveniently conducted in anhydrous ammonia.
[3] The THF-soluble triethylborane adduct of lithium sulfide can be generated using superhydride.